The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT)
- PMID: 22161728
- PMCID: PMC3427916
- DOI: 10.1002/jbmr.1494
The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT)
Erratum in
- J Bone Miner Res. 2012 Dec;27(12):2612
Abstract
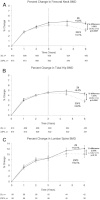
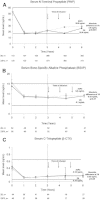
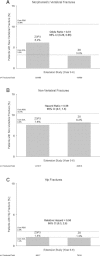
Zoledronic acid 5 mg (ZOL) annually for 3 years reduces fracture risk in postmenopausal women with osteoporosis. To investigate long-term effects of ZOL on bone mineral density (BMD) and fracture risk, the Health Outcomes and Reduced Incidence with Zoledronic acid Once Yearly-Pivotal Fracture Trial (HORIZON-PFT) was extended to 6 years. In this international, multicenter, double-blind, placebo-controlled extension trial, 1233 postmenopausal women who received ZOL for 3 years in the core study were randomized to 3 additional years of ZOL (Z6, n = 616) or placebo (Z3P3, n = 617). The primary endpoint was femoral neck (FN) BMD percentage change from year 3 to 6 in the intent-to-treat (ITT) population. Secondary endpoints included other BMD sites, fractures, biochemical bone turnover markers, and safety. In years 3 to 6, FN-BMD remained constant in Z6 and dropped slightly in Z3P3 (between-treatment difference = 1.04%; 95% confidence interval 0.4 to 1.7; p = 0.0009) but remained above pretreatment levels. Other BMD sites showed similar differences. Biochemical markers remained constant in Z6 but rose slightly in Z3P3, remaining well below pretreatment levels in both. New morphometric vertebral fractures were lower in the Z6 (n = 14) versus Z3P3 (n = 30) group (odds ratio = 0.51; p = 0.035), whereas other fractures were not different. Significantly more Z6 patients had a transient increase in serum creatinine >0.5 mg/dL (0.65% versus 2.94% in Z3P3). Nonsignificant increases in Z6 of atrial fibrillation serious adverse events (2.0% versus 1.1% in Z3P3; p = 0.26) and stroke (3.1% versus 1.5% in Z3P3; p = 0.06) were seen. Postdose symptoms were similar in both groups. Reports of hypertension were significantly lower in Z6 versus Z3P3 (7.8% versus 15.1%, p < 0.001). Small differences in bone density and markers in those who continued versus those who stopped treatment suggest residual effects, and therefore, after 3 years of annual ZOL, many patients may discontinue therapy up to 3 years. However, vertebral fracture reductions suggest that those at high fracture risk, particularly vertebral fracture, may benefit by continued treatment.
Trial registration: ClinicalTrials.gov NCT00145327.
Figures
Comment in
-
Bisphosphonate therapy for osteoporosis: the long and short of it.J Bone Miner Res. 2012 Feb;27(2):240-2. doi: 10.1002/jbmr.1542. J Bone Miner Res. 2012. PMID: 22271395 No abstract available.
References
-
- Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–22. - PubMed
-
- Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348(9041):1535–41. - PubMed
-
- Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280(24):2077–82. - PubMed
-
- Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH, 3rd, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282(14):1344–52. - PubMed
-
- McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, Adami S, Fogelman I, Diamond T, Eastell R, Meunier PJ, Reginster JY. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344(5):333–40. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

