Effect of oxidative stress on protein tyrosine phosphatase 1B in scleroderma dermal fibroblasts
- PMID: 22161819
- PMCID: PMC3309078
- DOI: 10.1002/art.34336
Effect of oxidative stress on protein tyrosine phosphatase 1B in scleroderma dermal fibroblasts
Abstract
Objective: Platelet-derived growth factor (PDGF) and its receptor, PDGFR, promote fibrosis in systemic sclerosis (SSc; scleroderma) dermal fibroblasts, and such cells in scleroderma skin lesions produce excessive reactive oxygen species (ROS). PDGFR is phosphorylated upon PDGF stimulation, and is dephosphorylated by protein tyrosine phosphatases (PTPs), including PTP1B. This study was undertaken to determine whether the thiol-sensitive PTP1B is affected by ROS in SSc dermal fibroblasts, thereby enhancing the phosphorylation of PDGFR and synthesis of type I collagen. This study also sought to investigate the effect of a thiol antioxidant, N-acetylcysteine (NAC), in SSc.
Methods: Fibroblasts were isolated from the skin of patients with diffuse SSc and normal healthy donors for cell culture experiments and immunofluorescence analyses. A phosphate release assay was used to determine the activity of PTP1B.
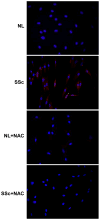
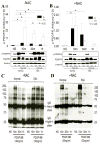
Results: Levels of ROS and type I collagen were significantly higher and amounts of free thiol were significantly lower in SSc fibroblasts compared to normal fibroblasts. After stimulation with PDGF, not only were PDGFR and ERK-1/2 phosphorylated to a greater extent, but also the ability to produce PTP1B was hampered in SSc fibroblasts. The activity of PTP1B was significantly inactivated in SSc fibroblasts as a result of cysteine oxidation by the raised levels of ROS, which was confirmed by the oxidation of multiple PTPs, including PTP1B, in SSc fibroblasts. Decreased expression of PTP1B in normal fibroblasts led to increased expression of type I collagen. Treatment of the cells with NAC restored the activity of PTP1B, improved the profile of PDGFR phosphorylation, decreased the numbers of tyrosine-phosphorylated proteins and levels of type I collagen, and scavenged ROS in SSc fibroblasts.
Conclusion: This study describes a new mechanism by which ROS may promote a profibrotic phenotype in SSc fibroblasts through the oxidative inactivation of PTP1B, leading to pronounced activation of PDGFR. The study also presents a novel molecular mechanism by which NAC may act on ROS and PTP1B to provide therapeutic benefit in SSc.
Copyright © 2012 by the American College of Rheumatology.
Figures

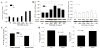


 : oxidation;
: oxidation;
 : less oxidation;
: less oxidation;
 : dephosphorylation;
: dephosphorylation;
 : unable to dephosphorylate
: unable to dephosphorylateReferences
-
- Jelaska A, Korn JH. Role of apoptosis and transforming growth factor beta1 in fibroblast selection and activation in systemic sclerosis. Arthritis Rheum. 2000;43:2230–9. - PubMed
-
- Pandolfi A, Florita M, Altomare G, Pigatto P, Donati MB, Poggi A. Increased plasma levels of platelet-derived growth factor activity in patients with progressive systemic sclerosis. Proc Soc Exp Biol Med. 1989;191:1–4. - PubMed
-
- Gay S, Jones RE, Jr, Huang GQ, Gay RE. Immunohistologic demonstration of platelet-derived growth factor (PDGF) and sis-oncogene expression in scleroderma. J Invest Dermatol. 1989;92:301–3. - PubMed
-
- Klareskog L, Gustafsson R, Scheynius A, Hallgren R. Increased expression of platelet-derived growth factor type B receptors in the skin of patients with systemic sclerosis. Arthritis Rheum. 1990;33:1534–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

