Simultaneous measurement of cerebral blood flow and transit time with turbo dynamic arterial spin labeling (Turbo-DASL): application to functional studies
- PMID: 22162211
- PMCID: PMC3309113
- DOI: 10.1002/mrm.23294
Simultaneous measurement of cerebral blood flow and transit time with turbo dynamic arterial spin labeling (Turbo-DASL): application to functional studies
Abstract
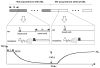
A turbo dynamic arterial spin labeling method (Turbo-DASL) was developed to simultaneously measure cerebral blood flow (CBF) and blood transit time with high temporal resolution. With Turbo-DASL, images were repeatedly acquired with a spiral readout after small-angle excitations during pseudocontinuous arterial spin labeling and control periods. Turbo-DASL experiments at 9.4 T without and with diffusion gradients were performed on rats anesthetized with isoflurane or α-chloralose. We determined blood transit times from carotid arteries to cortical arterial vessels (TT(a) ) from data obtained without diffusion gradients and to capillaries (TT(c) ) from data obtained with diffusion gradients. Cerebral arterial blood volume (CBV(a) ) was also calculated. At the baseline condition, both CBF and CBV(a) in the somatosensory cortical area were 40-50% less in rats with α-chloralose than in rats with isoflurane, while TT(a) and TT(c) were similar for both anesthetics. Absolute CBF and CBV(a) were positively correlated, while CBF and TT(c) were slightly negatively correlated. During forepaw stimulation, CBF increase was 15 ± 3% (n = 7) vs. 60 ± 7% (n = 5), and CBV(a) increase was 19 ± 9% vs. 46 ± 17% under isoflurane vs. α-chloralose anesthesia, respectively; CBF vs. CBV(a) changes were highly correlated. However, TT(a) and TT(c) were not significantly changed during stimulation. Our results support that arterial CBV increase plays a major role in functional CBF changes.
Copyright © 2011 Wiley Periodicals, Inc.
Figures





References
-
- Kim SG. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn Reson Med. 1995;34:293–301. - PubMed
-
- Edelman RR, Chen Q. EPISTAR MRI: multislice mapping of cerebral blood flow. Magn Reson Med. 1998;40:800–805. - PubMed
-
- Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II) Magn Reson Med. 1998;39:702–708. - PubMed
-
- Alsop DC, Detre JA. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab. 1996;16:1236–1249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

