Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841
- PMID: 22162570
- PMCID: PMC3269953
- DOI: 10.1200/JCO.2011.37.1039
Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841
Abstract
Purpose: Symptoms and complications of metastatic colorectal cancer (mCRC) differ by metastatic sites. There is a paucity of prospective survival data for patients with peritoneal carcinomatosis colorectal cancer (pcCRC). We characterized outcomes of patients with pcCRC enrolled onto two prospective randomized trials of chemotherapy and contrasted that with other manifestations of mCRC (non-pcCRC).
Methods: A total of 2,095 patients enrolled onto two prospective randomized trials were evaluated for overall survival (OS) and progression-free survival (PFS). A Cox proportional hazard model was used to assess the adjusted associations.
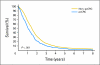
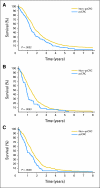
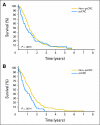
Results: The characteristics of the pcCRC group (n = 364) were similar to those of the non-pcCRC patients in median age (63 v 61 years, P = .23), sex (57% males v 61%, P = .23), and performance status (Eastern Cooperative Oncology Group performance status 0 or 1 94% v 96%, P = .06), but differed in frequency of liver (63% v 82%, P < .001) and lung metastases (27% v 34%, P = .01). Median OS (12.7 v 17.6 months, hazard ratio [HR] = 1.3; 95% CI, 1.2 to 1.5; P < .001) and PFS (5.8 v 7.2 months, HR = 1.2; 95% CI, 1.1 to 1.3; P = .001) were shorter for pcCRC versus non-pcCRC. The unfavorable prognostic influence of pcCRC remained after adjusting for age, PS, liver metastases, and other factors (OS: HR = 1.3, P < .001; PFS: HR = 1.1, P = .02). Infusional fluorouracil, leucovorin, and oxaliplatin was superior to irinotecan, leucovorin, and fluorouracil as a first-line treatment among pcCRC (HR for OS = 0.62, P = .005) and non-pcCRC patients (HR = 0.66, P < .001).
Conclusion: pcCRC is associated with a significantly shorter OS and PFS as compared with other manifestations of mCRC. Future trials for mCRC should consider stratifying on the basis of pcCRC status.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Factors dictating outcomes in patients with colorectal cancer and peritoneal carcinomatosis: selection, resection, or convection?J Clin Oncol. 2012 Jan 20;30(3):226-8. doi: 10.1200/JCO.2011.38.8199. Epub 2011 Dec 12. J Clin Oncol. 2012. PMID: 22162591 No abstract available.
-
Gastrointestinal cancer: Not all CRC subtypes are created equal.Nat Rev Clin Oncol. 2012 Jan 10;9(2):67. doi: 10.1038/nrclinonc.2011.203. Nat Rev Clin Oncol. 2012. PMID: 22231756 No abstract available.
-
Is the pooled analysis of the north central cancer treatment group phase III trials N9741 and N9841 biased?J Clin Oncol. 2012 May 20;30(15):1894-5; author reply 1895. doi: 10.1200/JCO.2012.41.9374. Epub 2012 Apr 2. J Clin Oncol. 2012. PMID: 22473158 No abstract available.
-
Prognosis of colorectal cancer with peritoneal carcinomatosis: is a new staging necessary?J Clin Oncol. 2012 Jun 20;30(18):2287-8; author reply 2288-9. doi: 10.1200/JCO.2012.42.1701. Epub 2012 May 21. J Clin Oncol. 2012. PMID: 22614990 No abstract available.
References
-
- Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. - PubMed
-
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

