Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986
- PMID: 22162575
- PMCID: PMC3255563
- DOI: 10.1200/JCO.2011.37.3571
Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986
Abstract
Purpose: This is the first randomized phase II/III trial comparing two carboplatin-based chemotherapy regimens in patients with urothelial cancer who are ineligible ("unfit") for cisplatin chemotherapy.
Patients and methods: The primary objective of the phase III part of this study was to compare the overall survival (OS) of chemotherapy-naive patients with measurable disease and an impaired renal function (glomerular filtration rate < 60 but > 30 mL/min) and/or performance score of 2 who were randomly assigned to receive either gemcitabine/carboplatin (GC) or methotrexate/carboplatin/vinblastine (M-CAVI). To detect an increase of 50% in median survival with GC compared with M-CAVI (13.5 v 9 months) based on a two-sided log-rank test at error rates α = .05 and β = .20, 225 patients were required. Secondary end points were overall response rate (ORR), progression-free survival (PFS), toxicity, and quality of life.
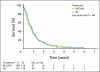
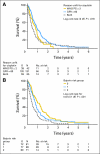
Results: In all, 238 patients were randomly assigned by 29 institutions over a period of 7 years. The median follow-up was 4.5 years. Best ORRs were 41.2% (36.1% confirmed response) for patients receiving GC versus 30.3% (21.0% confirmed response) for patients receiving M-CAVI (P = .08). Median OS was 9.3 months in the GC arm and 8.1 months in the M-CAVI arm (P = .64). There was no difference in PFS (P = .78) between the two arms. Severe acute toxicity (death, grade 4 thrombocytopenia with bleeding, grade 3 or 4 renal toxicity, neutropenic fever, or mucositis) was observed in 9.3% of patients receiving GC and 21.2% of patients receiving M-CAVI.
Conclusion: There were no significant differences in efficacy between the two treatment groups. The incidence of severe acute toxicities was higher for those receiving M-CAVI.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Re: randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC Study 30986.J Urol. 2012 May;187(5):1585-6. doi: 10.1016/j.juro.2012.01.105. Epub 2012 Mar 15. J Urol. 2012. PMID: 22494714 No abstract available.
References
-
- Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer. 2006;107:506–513. - PubMed
-
- Balducci L. Evidence-based management of cancer in the elderly. Cancer Control. 2000;7:368–376. - PubMed
-
- Balducci L, Extermann M. Management of cancer in the older person: A practical approach. Oncologist. 2000;5:224–237. - PubMed
-
- Balducci L, Yates J. General guidelines for the management of older patients with cancer. Oncology (Williston Park) 2000;14:221–227. - PubMed
-
- De Santis M, Bachner M. New developments in first- and second-line chemotherapy for transitional cell, squamous cell and adenocarcinoma of the bladder. Curr Opin Urol. 2007;17:363–368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

