Central and Peripheral GABA(A) Receptor Regulation of the Heart Rate Depends on the Conscious State of the Animal
- PMID: 22162673
- PMCID: PMC3226329
- DOI: 10.1155/2011/578273
Central and Peripheral GABA(A) Receptor Regulation of the Heart Rate Depends on the Conscious State of the Animal
Abstract
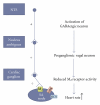
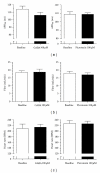
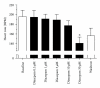
Intuitively one might expect that activation of GABAergic inhibitory neurons results in bradycardia. In conscious animals the opposite effect is however observed. GABAergic neurons in nucleus ambiguus hold the ability to control the activity of the parasympathetic vagus nerve that innervates the heart. Upon GABA activation the vagus nerve will be inhibited leaving less parasympathetic impact on the heart. The picture is however blurred in the presence of anaesthesia where both the concentration and type of anaesthetics can result in different effects on the cardiovascular system. This paper reviews cardiovascular outcomes of GABA activation and includes own experiments on anaesthetized animals and isolated hearts. In conclusion, the impact of changes in GABAergic input is very difficult to predict in these settings, emphasizing the need for experiments performed in conscious animals when aiming at determining the cardiovascular effects of compounds acting on GABAergic neurons.
Figures


References
-
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature Reviews Neuroscience. 2005;6(4):312–324. - PubMed
-
- Studer R, Von Boehmer L, Haenggi T, et al. Alteration of GABAergic synapses and gephyrin clusters in the thalamic reticular nucleus of GABAA receptor α3 subunit-null mice. European Journal of Neuroscience. 2006;24(5):1307–1315. - PubMed
-
- Crestani F, Löw K, Keist R, Mandelli MJ, Möhler H, Rudolph U. Molecular targets for the myorelaxant action of diazepam. Molecular Pharmacology. 2001;59(3):442–445. - PubMed
-
- Jurd R, Arras M, Lambert S, et al. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. The FASEB Journal. 2003;17(2):250–252. - PubMed
LinkOut - more resources
Full Text Sources

