Challenges posed to the maternal circulation by pregnancy
- PMID: 22162938
- PMCID: PMC3234126
- DOI: 10.2147/IBPC.S8393
Challenges posed to the maternal circulation by pregnancy
Abstract
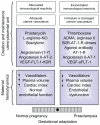
In primates, adequate growth of the fetus depends on the development of the uteroplacental unit. On the fetal side, this is achieved by the creation of the vascular network of the placenta. On the maternal side, the transformation of the spiral arteries into saccular nonreactive vessels by the trophoblast provides high blood flow to the intervillous space. Apart from the changes in the uterine arteries, the mother expands her plasma volume - at the expense of stimulating the renin-angiotensin-aldosterone system - and her cardiac output. In the maintaining of normotension in the face of an increased cardiac output and plasma volume, the renin-angiotensin-aldosterone system requires an enhanced vasodilator synthesis. Finally, in the late stages of pregnancy, a normal endothelial function is required to provide an ample margin to the activation provoked by deportation of syncytiotrophoblast fragments/factors to the maternal circulation. These four adaptative processes require various interrelated vasodilator systems. Deficient adaptations cause isolated or proteinuric arterial hypertension, intrauterine growth restriction, preterm delivery, and stillbirths, among others. Moreover, a normal or a defective adaptation to pregnancy influences maternal cardiovascular health in later life, as evidenced by various studies, most of them epidemiological; thus, pregnancy is now considered a stress test to the maternal cardiovascular system. Because of this, women planning to become pregnant should be screened for clinical and biochemical cardiovascular risks. Inversely, women presenting with hypertension in pregnancy should be thoroughly studied to detect and correct cardiovascular risks. The incorporation of the predictive value of a hypertensive pregnancy should help reduce cardiovascular disease in women.
Keywords: RAS; VEGF; kallikrein-kinin system; prostanoids; renin-angiotensin-aldosterone system.
Figures



Similar articles
-
Placental bed research: I. The placental bed: from spiral arteries remodeling to the great obstetrical syndromes.Am J Obstet Gynecol. 2019 Nov;221(5):437-456. doi: 10.1016/j.ajog.2019.05.044. Epub 2019 Jun 1. Am J Obstet Gynecol. 2019. PMID: 31163132 Review.
-
Placental bed research: II. Functional and immunological investigations of the placental bed.Am J Obstet Gynecol. 2019 Nov;221(5):457-469. doi: 10.1016/j.ajog.2019.07.010. Epub 2019 Jul 6. Am J Obstet Gynecol. 2019. PMID: 31288009 Review.
-
Uteroplacental hemostasis in intrauterine fetal growth retardation.Semin Thromb Hemost. 1999;25(5):443-6. doi: 10.1055/s-2007-994947. Semin Thromb Hemost. 1999. PMID: 10625199 Review.
-
Vasodilator factors in the systemic and local adaptations to pregnancy.Reprod Biol Endocrinol. 2009 Jul 31;7:79. doi: 10.1186/1477-7827-7-79. Reprod Biol Endocrinol. 2009. PMID: 19646248 Free PMC article. Review.
-
Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta.Dev Biol. 2002 Oct 15;250(2):358-73. doi: 10.1016/s0012-1606(02)90773-6. Dev Biol. 2002. PMID: 12376109
Cited by
-
Evaluation of placental oxygenation by near-infrared spectroscopy in relation to ultrasound maturation grade in physiological term pregnancies.Open Med (Wars). 2023 Nov 7;18(1):20230843. doi: 10.1515/med-2023-0843. eCollection 2023. Open Med (Wars). 2023. PMID: 38025545 Free PMC article.
-
Current Updates on Pre-eclampsia: Maternal and Foetal Cardiovascular Diseases Predilection, Science or Myth? : Future cardiovascular disease risks in mother and child following pre-eclampsia.Curr Hypertens Rep. 2021 Mar 10;23(3):16. doi: 10.1007/s11906-021-01132-x. Curr Hypertens Rep. 2021. PMID: 33694011 Review.
-
Photoacoustic imaging for in vivo quantification of placental oxygenation in mice.FASEB J. 2017 Dec;31(12):5520-5529. doi: 10.1096/fj.201700047RR. Epub 2017 Aug 21. FASEB J. 2017. PMID: 28842425 Free PMC article.
-
The hemodynamic basis for positional- and inter-fetal dependent effects in dual arterial supply of mouse pregnancies.PLoS One. 2012;7(12):e52273. doi: 10.1371/journal.pone.0052273. Epub 2012 Dec 20. PLoS One. 2012. PMID: 23284965 Free PMC article.
-
Plasticity of the Maternal Vasculature During Pregnancy.Annu Rev Physiol. 2019 Feb 10;81:89-111. doi: 10.1146/annurev-physiol-020518-114435. Annu Rev Physiol. 2019. PMID: 30742784 Free PMC article. Review.
References
-
- Charnock-Jones DS, Kaufmann P, Mayhew TM. Aspects of human fetoplacental vasculogenesis and angiogenesis. I. Molecular regulation. Placenta. 2004;25:103–113. - PubMed
-
- Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69:1–7. - PubMed
-
- Hytten F. Blood volume changes in normal pregnancy. Clin Haematol. 1985;14:601–612. - PubMed
-
- Salas SP, Rosso P, Espinoza R, Robert JA, Valdés G, Donoso E. Maternal plasma volume expansion and hormonal changes in women with idiopathic fetal growth retardation. Obstet Gynecol. 1993;81:1029–1033. - PubMed
LinkOut - more resources
Full Text Sources

