The cost-effectiveness and value of information of three influenza vaccination dosing strategies for individuals with human immunodeficiency virus
- PMID: 22162988
- PMCID: PMC3232195
- DOI: 10.1371/journal.pone.0027059
The cost-effectiveness and value of information of three influenza vaccination dosing strategies for individuals with human immunodeficiency virus
Abstract
Background: Influenza vaccine immunogenicity is diminished in patients living with HIV/AIDS. We evaluated the cost-effectiveness and expected value of perfect information (EVPI) of three alternative influenza vaccine dosing strategies intended to increase immunogenicity in those patients.
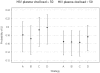
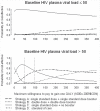
Methods: A randomized, multi-centered, controlled, vaccine trial was conducted at 12 CIHR Canadian HIV Trials Network sites. Three dosing strategies with seasonal, inactivated trivalent, non-adjuvanted intramuscular vaccine were used in HIV infected adults: two standard doses over 28 days (Strategy A), two double doses over 28 days (Strategy B) and a single standard dose of influenza vaccine (Strategy C), administered prior to the 2008 influenza season. The comparator in our analysis was practice in the previous year, in which 82.8% of HIV/AIDS received standard-dose vaccination (Strategy D). A Markov cohort model was developed to estimate the monthly probability of Influenza-like Illness (ILI) over one influenza season. Costs and quality-adjusted life years, extrapolated to the lifetime of the hypothetical study cohorts, were estimated in calculating incremental cost-effectiveness ratios (ICER) and EVPI in conducting further research.
Results: 298 patients with median CD4 of 470 cells/µl and 76% with viral load suppression were randomized. Strategy C was the most cost-effective strategy for the overall trial population and for suppressed and unsuppressed individuals. Mean ICERs for Strategy A for unsuppressed patients could also be considered cost-effective. The level of uncertainty regarding the decision to implement strategy A versus C for unsuppressed individuals was high. The maximum acceptable cost of reducing decision uncertainty in implementing strategy A for individuals with unsuppressed pVL was $418,000--below the cost of conducting a larger-scale trial.
Conclusion: Our results do not support a policy to implement increased antigen dose or booster dosing strategies with seasonal, inactivated trivalent, non-adjuvanted intramuscular vaccine for individuals with HIV in Canada.
Trial registration: ClinicalTrials.gov NCT00764998.
Conflict of interest statement
Figures


References
-
- Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). CDC weekly report. 99; 48(RR-4):1–28. Available from www.cdc.gov. Accessed March 2010. - PubMed
-
- Lin JC, Nichol KL. Excess mortality due to pneumonia or influenza during influenza seasons among persons with acquired immunodeficiency syndrome. Arch Intern Med. 2001;161:441–446. - PubMed
-
- Fine AD, Bridges CB, De Guzman AM, Glover L, Zeller B, et al. Influenza A among patients with human immunodeficiency virus: an outbreak of infection at a residential facility in New York City. Clin Infect Dis. 2001;32(12):1784–91. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

