Cannabidiol reduces intestinal inflammation through the control of neuroimmune axis
- PMID: 22163000
- PMCID: PMC3232190
- DOI: 10.1371/journal.pone.0028159
Cannabidiol reduces intestinal inflammation through the control of neuroimmune axis
Abstract
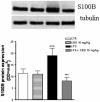
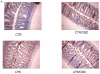
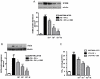
Enteric glial cells (EGC) actively mediate acute and chronic inflammation in the gut; EGC proliferate and release neurotrophins, growth factors, and pro-inflammatory cytokines which, in turn, may amplify the immune response, representing a very important link between the nervous and immune systems in the intestine. Cannabidiol (CBD) is an interesting compound because of its ability to control reactive gliosis in the CNS, without any unwanted psychotropic effects. Therefore the rationale of our study was to investigate the effect of CBD on intestinal biopsies from patients with ulcerative colitis (UC) and from intestinal segments of mice with LPS-induced intestinal inflammation. CBD markedly counteracted reactive enteric gliosis in LPS-mice trough the massive reduction of astroglial signalling neurotrophin S100B. Histological, biochemical and immunohistochemical data demonstrated that S100B decrease was associated with a considerable decrease in mast cell and macrophages in the intestine of LPS-treated mice after CBD treatment. Moreover the treatment of LPS-mice with CBD reduced TNF-α expression and the presence of cleaved caspase-3. Similar results were obtained in ex vivo cultured human derived colonic biopsies. In biopsies of UC patients, both during active inflammation and in remission stimulated with LPS+INF-γ, an increased glial cell activation and intestinal damage were evidenced. CBD reduced the expression of S100B and iNOS proteins in the human biopsies confirming its well documented effect in septic mice. The activity of CBD is, at least partly, mediated via the selective PPAR-gamma receptor pathway. CBD targets enteric reactive gliosis, counteracts the inflammatory environment induced by LPS in mice and in human colonic cultures derived from UC patients. These actions lead to a reduction of intestinal damage mediated by PPARgamma receptor pathway. Our results therefore indicate that CBD indeed unravels a new therapeutic strategy to treat inflammatory bowel diseases.
Conflict of interest statement
Figures

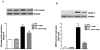



References
-
- Ben-Horin S, Chowers Y. Neuroimmunology of the gut: physiology, pathology, and pharmacology. Curr Opin Pharmacol. 2008;8:490–5. - PubMed
-
- Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136:2003–14. - PubMed
-
- Qiao S, Iwashita T, Ichihara M. Increased expression of glial cell line-derived neurotrophic factor and neurturin in a case of colon adenocarcinoma associated with diffuse ganglioneuromatosis. Clin Neuropathol. 2009;28:105–12. - PubMed
-
- Burns AJ, Pachnis V. Development of the enteric nervous system: bringing together cells, signals and genes. Neurogastroenterol Motil. 2009;21:100–2. Review. - PubMed
-
- Esposito G, Cirillo C, Sarnelli G, De Filippis D, Iuvone T, et al. Enteric glial-derived S100B protein stimulates nitric oxide production in celiac disease. Gastroenterology. 2007;133:918–25. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

