Correlation of inter-locus polyglutamine toxicity with CAG•CTG triplet repeat expandability and flanking genomic DNA GC content
- PMID: 22163004
- PMCID: PMC3232215
- DOI: 10.1371/journal.pone.0028260
Correlation of inter-locus polyglutamine toxicity with CAG•CTG triplet repeat expandability and flanking genomic DNA GC content
Abstract
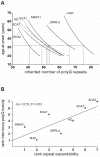
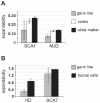
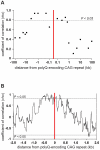
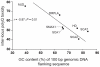
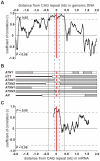
Dynamic expansions of toxic polyglutamine (polyQ)-encoding CAG repeats in ubiquitously expressed, but otherwise unrelated, genes cause a number of late-onset progressive neurodegenerative disorders, including Huntington disease and the spinocerebellar ataxias. As polyQ toxicity in these disorders increases with repeat length, the intergenerational expansion of unstable CAG repeats leads to anticipation, an earlier age-at-onset in successive generations. Crucially, disease associated alleles are also somatically unstable and continue to expand throughout the lifetime of the individual. Interestingly, the inherited polyQ length mediating a specific age-at-onset of symptoms varies markedly between disorders. It is widely assumed that these inter-locus differences in polyQ toxicity are mediated by protein context effects. Previously, we demonstrated that the tendency of expanded CAG•CTG repeats to undergo further intergenerational expansion (their 'expandability') also differs between disorders and these effects are strongly correlated with the GC content of the genomic flanking DNA. Here we show that the inter-locus toxicity of the expanded polyQ tracts of these disorders also correlates with both the expandability of the underlying CAG repeat and the GC content of the genomic DNA flanking sequences. Inter-locus polyQ toxicity does not correlate with properties of the mRNA or protein sequences, with polyQ location within the gene or protein, or steady state transcript levels in the brain. These data suggest that the observed inter-locus differences in polyQ toxicity are not mediated solely by protein context effects, but that genomic context is also important, an effect that may be mediated by modifying the rate at which somatic expansion of the DNA delivers proteins to their cytotoxic state.
Conflict of interest statement
Figures
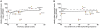
Similar articles
-
Age-, tissue- and length-dependent bidirectional somatic CAG•CTG repeat instability in an allelic series of R6/2 Huntington disease mice.Neurobiol Dis. 2015 Apr;76:98-111. doi: 10.1016/j.nbd.2015.01.004. Epub 2015 Feb 3. Neurobiol Dis. 2015. PMID: 25662336
-
The CAG/polyglutamine tract diseases: gene products and molecular pathogenesis.Brain Pathol. 1997 Jul;7(3):927-42. doi: 10.1111/j.1750-3639.1997.tb00894.x. Brain Pathol. 1997. PMID: 9217976 Free PMC article. Review.
-
Cis-acting modifiers of expanded CAG/CTG triplet repeat expandability: associations with flanking GC content and proximity to CpG islands.Hum Mol Genet. 1999 Jun;8(6):1061-7. doi: 10.1093/hmg/8.6.1061. Hum Mol Genet. 1999. PMID: 10332038
-
Spinocerebellar ataxia type 2: polyQ repeat variation in the CACNA1A calcium channel modifies age of onset.Brain. 2005 Oct;128(Pt 10):2297-303. doi: 10.1093/brain/awh586. Epub 2005 Jul 6. Brain. 2005. PMID: 16000334
-
Preclinical Nanomedicines for Polyglutamine-Based Neurodegenerative Diseases.Mol Pharm. 2021 Feb 1;18(2):610-626. doi: 10.1021/acs.molpharmaceut.0c00506. Epub 2020 Jul 10. Mol Pharm. 2021. PMID: 32584043 Review.
Cited by
-
RNA toxicity in polyglutamine disorders: concepts, models, and progress of research.J Mol Med (Berl). 2013 Jun;91(6):683-91. doi: 10.1007/s00109-013-1016-2. Epub 2013 Mar 20. J Mol Med (Berl). 2013. PMID: 23512265 Free PMC article. Review.
-
CAG repeat mosaicism is gene specific in spinocerebellar ataxias.Am J Hum Genet. 2024 May 2;111(5):913-926. doi: 10.1016/j.ajhg.2024.03.015. Epub 2024 Apr 15. Am J Hum Genet. 2024. PMID: 38626762 Free PMC article.
-
Repeat instability during DNA repair: Insights from model systems.Crit Rev Biochem Mol Biol. 2015 Mar-Apr;50(2):142-67. doi: 10.3109/10409238.2014.999192. Epub 2015 Jan 22. Crit Rev Biochem Mol Biol. 2015. PMID: 25608779 Free PMC article. Review.
-
Modifiers of CAG/CTG Repeat Instability: Insights from Mammalian Models.J Huntingtons Dis. 2021;10(1):123-148. doi: 10.3233/JHD-200426. J Huntingtons Dis. 2021. PMID: 33579861 Free PMC article. Review.
-
A pan-European study of the C9orf72 repeat associated with FTLD: geographic prevalence, genomic instability, and intermediate repeats.Hum Mutat. 2013 Feb;34(2):363-73. doi: 10.1002/humu.22244. Epub 2013 Jan 4. Hum Mutat. 2013. PMID: 23111906 Free PMC article.
References
-
- Gomes-Pereira M, Monckton DG. Chemical modifiers of unstable expanded simple sequence repeats: what goes up, could come down. Mutat Res. 2006;598:15–34. - PubMed
-
- Gusella JF, MacDonald ME. Molecular genetics: unmasking polyglutamine triggers in neurodegenerative disease. Nat Rev Neurosci. 2000;1:109–115. - PubMed
-
- Richards RI, Sutherland GR. Heritable unstable DNA sequences. Nat Genet. 1992;1:7–9. - PubMed
-
- Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67:1047–1058. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

