MicroRNA-1 and -133 increase arrhythmogenesis in heart failure by dissociating phosphatase activity from RyR2 complex
- PMID: 22163007
- PMCID: PMC3232211
- DOI: 10.1371/journal.pone.0028324
MicroRNA-1 and -133 increase arrhythmogenesis in heart failure by dissociating phosphatase activity from RyR2 complex
Abstract
In heart failure (HF), arrhythmogenic spontaneous sarcoplasmic reticulum (SR) Ca(2+) release and afterdepolarizations in cardiac myocytes have been linked to abnormally high activity of ryanodine receptors (RyR2s) associated with enhanced phosphorylation of the channel. However, the specific molecular mechanisms underlying RyR2 hyperphosphorylation in HF remain poorly understood. The objective of the current study was to test the hypothesis that the enhanced expression of muscle-specific microRNAs (miRNAs) underlies the HF-related alterations in RyR2 phosphorylation in ventricular myocytes by targeting phosphatase activity localized to the RyR2. We studied hearts isolated from canines with chronic HF exhibiting increased left ventricular (LV) dimensions and decreased LV contractility. qRT-PCR revealed that the levels of miR-1 and miR-133, the most abundant muscle-specific miRNAs, were significantly increased in HF myocytes compared with controls (2- and 1.6-fold, respectively). Western blot analyses demonstrated that expression levels of the protein phosphatase 2A (PP2A) catalytic and regulatory subunits, which are putative targets of miR-133 and miR-1, were decreased in HF cells. PP2A catalytic subunit mRNAs were validated as targets of miR-133 by using luciferase reporter assays. Pharmacological inhibition of phosphatase activity increased the frequency of diastolic Ca(2+) waves and afterdepolarizations in control myocytes. The decreased PP2A activity observed in HF was accompanied by enhanced Ca(2+)/calmodulin-dependent protein kinase (CaMKII)-mediated phosphorylation of RyR2 at sites Ser-2814 and Ser-2030 and increased frequency of diastolic Ca(2+) waves and afterdepolarizations in HF myocytes compared with controls. In HF myocytes, CaMKII inhibitory peptide normalized the frequency of pro-arrhythmic spontaneous diastolic Ca(2+) waves. These findings suggest that altered levels of major muscle-specific miRNAs contribute to abnormal RyR2 function in HF by depressing phosphatase activity localized to the channel, which in turn, leads to the excessive phosphorylation of RyR2s, abnormal Ca(2+) cycling, and increased propensity to arrhythmogenesis.
Conflict of interest statement
Figures
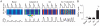


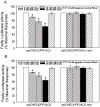



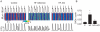
References
-
- Mozaffarian D, Anker SD, Anand I, Linker DT, Sullivan MD, et al. Prediction of mode of death in heart failure: the Seattle Heart Failure Model. Circulation. 2007;116:392–398. - PubMed
-
- Janse MJ. Electrophysiological changes in heart failure and their relationship to arrhythmogenesis. Cardiovasc Res. 2004;61:208–217. - PubMed
-
- Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med. 2004;14:61–66. - PubMed
-
- Hasenfuss G, Pieske B. Calcium cycling in congestive heart failure. J Mol Cell Cardiol. 2002;34:951–969. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL089836/HL/NHLBI NIH HHS/United States
- 5T32HL094300/HL/NHLBI NIH HHS/United States
- HL074045/HL/NHLBI NIH HHS/United States
- R21 HD058997/HD/NICHD NIH HHS/United States
- T32 HL094300/HL/NHLBI NIH HHS/United States
- R01 HL048848/HL/NHLBI NIH HHS/United States
- R01 HL074045/HL/NHLBI NIH HHS/United States
- R01 HL089836/HL/NHLBI NIH HHS/United States
- R29 HL048848/HL/NHLBI NIH HHS/United States
- HD058997/HD/NICHD NIH HHS/United States
- HL048848/HL/NHLBI NIH HHS/United States
- R01 HL063043/HL/NHLBI NIH HHS/United States
- HL063043/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

