IL-1α/IL-1R1 expression in chronic obstructive pulmonary disease and mechanistic relevance to smoke-induced neutrophilia in mice
- PMID: 22163019
- PMCID: PMC3232226
- DOI: 10.1371/journal.pone.0028457
IL-1α/IL-1R1 expression in chronic obstructive pulmonary disease and mechanistic relevance to smoke-induced neutrophilia in mice
Abstract
Background: Cigarette smoking is the main risk factor for the development of chronic obstructive pulmonary disease (COPD), a major cause of morbidity and mortality worldwide. Despite this, the cellular and molecular mechanisms that contribute to COPD pathogenesis are still poorly understood.
Methodology and principal findings: The objective of this study was to assess IL-1 α and β expression in COPD patients and to investigate their respective roles in perpetuating cigarette smoke-induced inflammation. Functional studies were pursued in smoke-exposed mice using gene-deficient animals, as well as blocking antibodies for IL-1α and β. Here, we demonstrate an underappreciated role for IL-1α expression in COPD. While a strong correlation existed between IL-1α and β levels in patients during stable disease and periods of exacerbation, neutrophilic inflammation was shown to be IL-1α-dependent, and IL-1β- and caspase-1-independent in a murine model of cigarette smoke exposure. As IL-1α was predominantly expressed by hematopoietic cells in COPD patients and in mice exposed to cigarette smoke, studies pursued in bone marrow chimeric mice demonstrated that the crosstalk between IL-1α+ hematopoietic cells and the IL-1R1+ epithelial cells regulates smoke-induced inflammation. IL-1α/IL-1R1-dependent activation of the airway epithelium also led to exacerbated inflammatory responses in H1N1 influenza virus infected smoke-exposed mice, a previously reported model of COPD exacerbation.
Conclusions and significance: This study provides compelling evidence that IL-1α is central to the initiation of smoke-induced neutrophilic inflammation and suggests that IL-1α/IL-1R1 targeted therapies may be relevant for limiting inflammation and exacerbations in COPD.
Conflict of interest statement
Figures



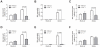


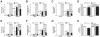
References
-
- Patel RR, Ryu JH, Vassallo R. Cigarette smoking and diffuse lung disease. Drugs. 2008:1511–1527. - PubMed
-
- Holmes J. 10 Facts on the Tobacco Epidemic and Global Tobacco Control. World Health Organization; 2010.
-
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009:519–550. - PubMed
-
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010:821–832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

