Selective coupling between theta phase and neocortical fast gamma oscillations during REM-sleep in mice
- PMID: 22163023
- PMCID: PMC3230633
- DOI: 10.1371/journal.pone.0028489
Selective coupling between theta phase and neocortical fast gamma oscillations during REM-sleep in mice
Abstract
Background: The mammalian brain expresses a wide range of state-dependent network oscillations which vary in frequency and spatial extension. Such rhythms can entrain multiple neurons into coherent patterns of activity, consistent with a role in behaviour, cognition and memory formation. Recent evidence suggests that locally generated fast network oscillations can be systematically aligned to long-range slow oscillations. It is likely that such cross-frequency coupling supports specific tasks including behavioural choice and working memory.
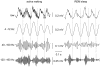
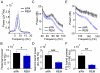
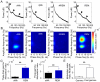
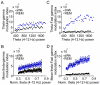
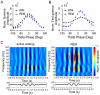
Principal findings: We analyzed temporal coupling between high-frequency oscillations and EEG theta activity (4-12 Hz) in recordings from mouse parietal neocortex. Theta was exclusively present during active wakefulness and REM-sleep. Fast oscillations occurred in two separate frequency bands: gamma (40-100 Hz) and fast gamma (120-160 Hz). Theta, gamma and fast gamma were more prominent during active wakefulness as compared to REM-sleep. Coupling between theta and the two types of fast oscillations, however, was more pronounced during REM-sleep. This state-dependent cross-frequency coupling was particularly strong for theta-fast gamma interaction which increased 9-fold during REM as compared to active wakefulness. Theta-gamma coupling increased only by 1.5-fold.
Significance: State-dependent cross-frequency-coupling provides a new functional characteristic of REM-sleep and establishes a unique property of neocortical fast gamma oscillations. Interactions between defined patterns of slow and fast network oscillations may serve selective functions in sleep-dependent information processing.
Conflict of interest statement
Figures
Similar articles
-
Distinct features of fast oscillations in phasic and tonic rapid eye movement sleep.J Sleep Res. 2012 Dec;21(6):630-3. doi: 10.1111/j.1365-2869.2012.01037.x. Epub 2012 Jul 20. J Sleep Res. 2012. PMID: 22812730
-
Dynamic modulation of theta-gamma coupling during rapid eye movement sleep.Sleep. 2019 Dec 24;42(12):zsz182. doi: 10.1093/sleep/zsz182. Sleep. 2019. PMID: 31410477
-
Theta-gamma coupling during REM sleep depends on breathing rate.Sleep. 2021 Dec 10;44(12):zsab189. doi: 10.1093/sleep/zsab189. Sleep. 2021. PMID: 34297128
-
Theta-associated high-frequency oscillations (110-160Hz) in the hippocampus and neocortex.Prog Neurobiol. 2013 Jan;100:1-14. doi: 10.1016/j.pneurobio.2012.09.002. Epub 2012 Sep 25. Prog Neurobiol. 2013. PMID: 23022096 Review.
-
Fast network oscillations during non-REM sleep support memory consolidation.Neurosci Res. 2023 Apr;189:3-12. doi: 10.1016/j.neures.2022.12.019. Epub 2022 Dec 26. Neurosci Res. 2023. PMID: 36581177 Review.
Cited by
-
The Sleep Side of Aging and Alzheimer's Disease.Sleep Med. 2021 Jan;77:209-225. doi: 10.1016/j.sleep.2020.05.029. Epub 2020 May 30. Sleep Med. 2021. PMID: 32912799 Free PMC article. Review.
-
In vivo assessment of behavioral recovery and circulatory exchange in the peritoneal parabiosis model.Sci Rep. 2016 Jul 1;6:29015. doi: 10.1038/srep29015. Sci Rep. 2016. PMID: 27364522 Free PMC article.
-
Mechanisms of neural organization and rhythmogenesis during hippocampal and cortical ripples.Philos Trans R Soc Lond B Biol Sci. 2020 May 25;375(1799):20190237. doi: 10.1098/rstb.2019.0237. Epub 2020 Apr 6. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 32248777 Free PMC article. Review.
-
Dopamine Modulates Delta-Gamma Phase-Amplitude Coupling in the Prefrontal Cortex of Behaving Rats.Front Neural Circuits. 2017 May 9;11:29. doi: 10.3389/fncir.2017.00029. eCollection 2017. Front Neural Circuits. 2017. PMID: 28536507 Free PMC article.
-
Impaired θ-γ Coupling Indicates Inhibitory Dysfunction and Seizure Risk in a Dravet Syndrome Mouse Model.J Neurosci. 2021 Jan 20;41(3):524-537. doi: 10.1523/JNEUROSCI.2132-20.2020. Epub 2020 Nov 24. J Neurosci. 2021. PMID: 33234612 Free PMC article.
References
-
- Berger H. Über das Elektrenkephalogramm des Menschen. Arch Psychiat Nervenkrankh. 1929;87:527–570.
-
- Singer W. Synchronization of cortical activity and its putative role in information processing and learning. Annu Rev Physiol. 1993;55:349–374. - PubMed
-
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. - PubMed
-
- Jensen O, Kaiser J, Lachaux J-P. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30:317–324. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

