Tyrosine phosphorylation of Rac1: a role in regulation of cell spreading
- PMID: 22163037
- PMCID: PMC3232246
- DOI: 10.1371/journal.pone.0028587
Tyrosine phosphorylation of Rac1: a role in regulation of cell spreading
Abstract
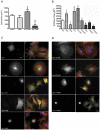
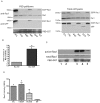
Rac1 influences a multiplicity of vital cellular- and tissue-level control functions, making it an important candidate for targeted therapeutics. The activity of the Rho family member Cdc42 has been shown to be modulated by tyrosine phosphorylation at position 64. We therefore investigated consequences of the point mutations Y64F and Y64D in Rac1. Both mutations altered cell spreading from baseline in the settings of wild type, constitutively active, or dominant negative Rac1 expression, and were accompanied by differences in Rac1 targeting to focal adhesions. Rac1-Y64F displayed increased GTP-binding, increased association with βPIX, and reduced binding with RhoGDI as compared with wild type Rac1. Rac1-Y64D had less binding to PAK than Rac1-WT or Rac1-64F. In vitro assays demonstrated that Y64 in Rac1 is a target for FAK and Src. Taken together, these data suggest a mechanism for the regulation of Rac1 activity by non-receptor tyrosine kinases, with consequences for membrane extension.
Conflict of interest statement
Figures








Similar articles
-
FAK potentiates Rac1 activation and localization to matrix adhesion sites: a role for betaPIX.Mol Biol Cell. 2007 Jan;18(1):253-64. doi: 10.1091/mbc.e06-03-0207. Epub 2006 Nov 8. Mol Biol Cell. 2007. PMID: 17093062 Free PMC article.
-
Regulation of ephexin1, a guanine nucleotide exchange factor of Rho family GTPases, by fibroblast growth factor receptor-mediated tyrosine phosphorylation.J Biol Chem. 2007 Oct 19;282(42):31103-12. doi: 10.1074/jbc.M704430200. Epub 2007 Aug 16. J Biol Chem. 2007. PMID: 17702745
-
p85 beta-PIX is required for cell motility through phosphorylations of focal adhesion kinase and p38 MAP kinase.Exp Cell Res. 2005 Jul 15;307(2):315-28. doi: 10.1016/j.yexcr.2005.03.028. Exp Cell Res. 2005. PMID: 15893751
-
Regulation of osteoclast apoptosis and motility by small GTPase binding protein Rac1.J Bone Miner Res. 2005 Dec;20(12):2245-53. doi: 10.1359/JBMR.050816. Epub 2005 Aug 22. J Bone Miner Res. 2005. PMID: 16294277
-
Hyperosmotic stress induces rapid focal adhesion kinase phosphorylation at tyrosines 397 and 577. Role of Src family kinases and Rho family GTPases.J Biol Chem. 2004 Oct 22;279(43):45266-78. doi: 10.1074/jbc.M314132200. Epub 2004 Aug 9. J Biol Chem. 2004. PMID: 15302877
Cited by
-
Caveolin-1 regulates Rac1 activation and rat pulmonary microvascular endothelial hyperpermeability induced by TNF-α.PLoS One. 2013;8(1):e55213. doi: 10.1371/journal.pone.0055213. Epub 2013 Jan 30. PLoS One. 2013. PMID: 23383114 Free PMC article.
-
Post-Translational Modification and Subcellular Distribution of Rac1: An Update.Cells. 2018 Dec 11;7(12):263. doi: 10.3390/cells7120263. Cells. 2018. PMID: 30544910 Free PMC article. Review.
-
PFTK1 kinase regulates axogenesis during development via RhoA activation.BMC Biol. 2023 Oct 31;21(1):240. doi: 10.1186/s12915-023-01732-w. BMC Biol. 2023. PMID: 37907898 Free PMC article.
-
ERK3/MAPK6 dictates CDC42/RAC1 activity and ARP2/3-dependent actin polymerization.Elife. 2023 Apr 14;12:e85167. doi: 10.7554/eLife.85167. Elife. 2023. PMID: 37057894 Free PMC article.
-
Rho GTPases, their post-translational modifications, disease-associated mutations and pharmacological inhibitors.Small GTPases. 2018 May 4;9(3):203-215. doi: 10.1080/21541248.2016.1218407. Epub 2016 Aug 22. Small GTPases. 2018. PMID: 27548350 Free PMC article. Review.
References
-
- Hordijk PL. Regulation of NADPH oxidases: the role of Rac proteins. Circ Res. 2006;98:453–462. - PubMed
-
- Kissil JL, Walmsley MJ, Hanlon L, Haigis KM, Bender Kim CF, et al. Requirement for Rac1 in a K-ras induced lung cancer in the mouse. Cancer Res. 2007;67:8089–8094. - PubMed
-
- Nikolova E, Mitev V, Zhelev N, Deroanne CF, Poumay Y. The small Rho GTPase Rac1 controls normal human dermal fibroblasts proliferation with phosphorylation of the oncoprotein c-myc. Biochem Biophys Res Commun. 2007;359:834–839. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

