Generation of variants in Listeria monocytogenes continuous-flow biofilms is dependent on radical-induced DNA damage and RecA-mediated repair
- PMID: 22163039
- PMCID: PMC3230620
- DOI: 10.1371/journal.pone.0028590
Generation of variants in Listeria monocytogenes continuous-flow biofilms is dependent on radical-induced DNA damage and RecA-mediated repair
Abstract
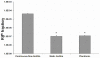
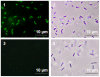
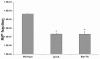
The food-borne pathogen Listeria monocytogenes is a gram-positive microaerophilic facultative anaerobic rod and the causative agent of the devastating disease listeriosis. L. monocytogenes is able to form biofilms in the food processing environment. Since biofilms are generally hard to eradicate, they can function as a source for food contamination. In several occasions biofilms have been identified as a source for genetic variability, which potentially can result in adaptation of strains to food processing or clinical conditions. However, nothing is known about mutagenesis in L. monocytogenes biofilms and the possible mechanisms involved. In this study, we showed that the generation of genetic variants was specifically induced in continuous-flow biofilms of L. monocytogenes, but not in static biofilms. Using specific dyes and radical inhibitors, we showed that the formation of superoxide and hydroxyl radicals was induced in continuous-flow biofilms, which was accompanied with in an increase in DNA damage. Promoter reporter studies showed that recA, which is an important component in DNA repair and the activator of the SOS response, is activated in continuous-flow biofilms and that activation was dependent on radical-induced DNA damage. Furthermore, continuous-flow biofilm experiments using an in-frame recA deletion mutant verified that RecA is required for induced generation of genetic variants. Therefore, we can conclude that generation of genetic variants in L. monocytogenes continuous-flow biofilms results from radical-induced DNA damage and RecA-mediated mutagenic repair of the damaged DNA.
Conflict of interest statement
Figures





Similar articles
-
The SOS response of Listeria monocytogenes is involved in stress resistance and mutagenesis.Microbiology (Reading). 2010 Feb;156(Pt 2):374-384. doi: 10.1099/mic.0.035196-0. Epub 2009 Nov 5. Microbiology (Reading). 2010. PMID: 19892760
-
Importance of SigB for Listeria monocytogenes static and continuous-flow biofilm formation and disinfectant resistance.Appl Environ Microbiol. 2010 Dec;76(23):7854-60. doi: 10.1128/AEM.01519-10. Epub 2010 Oct 1. Appl Environ Microbiol. 2010. PMID: 20889779 Free PMC article.
-
Molecular and functional characterization of the Listeria monocytogenes RecA protein: Insights into the homologous recombination process.Int J Biochem Cell Biol. 2020 Feb;119:105642. doi: 10.1016/j.biocel.2019.105642. Epub 2019 Nov 4. Int J Biochem Cell Biol. 2020. PMID: 31698090
-
[Advances on the mechanisms regulating the formation of the biofilm of Listeria monocytogenes].Sheng Wu Gong Cheng Xue Bao. 2021 Sep 25;37(9):3151-3161. doi: 10.13345/j.cjb.200734. Sheng Wu Gong Cheng Xue Bao. 2021. PMID: 34622624 Review. Chinese.
-
Listeria monocytogenes in Food-Processing Facilities, Food Contamination, and Human Listeriosis: The Brazilian Scenario.Foodborne Pathog Dis. 2017 Nov;14(11):623-636. doi: 10.1089/fpd.2016.2274. Epub 2017 Aug 2. Foodborne Pathog Dis. 2017. PMID: 28767285 Review.
Cited by
-
Concentration-dependent activity of antibiotics in natural environments.Front Microbiol. 2013 Feb 13;4:20. doi: 10.3389/fmicb.2013.00020. eCollection 2013. Front Microbiol. 2013. PMID: 23422936 Free PMC article.
-
Iron triggers λSo prophage induction and release of extracellular DNA in Shewanella oneidensis MR-1 biofilms.Appl Environ Microbiol. 2014 Sep;80(17):5304-16. doi: 10.1128/AEM.01480-14. Epub 2014 Jun 20. Appl Environ Microbiol. 2014. PMID: 24951794 Free PMC article.
-
Effect of arsenite and growth in biofilm conditions on the evolution of Thiomonas sp. CB2.Microb Genom. 2020 Oct;6(10):mgen000447. doi: 10.1099/mgen.0.000447. Microb Genom. 2020. PMID: 33034553 Free PMC article.
-
Reactive oxygen species in the signaling and adaptation of multicellular microbial communities.Oxid Med Cell Longev. 2012;2012:976753. doi: 10.1155/2012/976753. Epub 2012 Jul 1. Oxid Med Cell Longev. 2012. PMID: 22829965 Free PMC article. Review.
-
Nfu facilitates the maturation of iron-sulfur proteins and participates in virulence in Staphylococcus aureus.Mol Microbiol. 2015 Feb;95(3):383-409. doi: 10.1111/mmi.12860. Epub 2014 Dec 20. Mol Microbiol. 2015. PMID: 25388433 Free PMC article.
References
-
- Pritchard TJ, Flanders KJ, Donnelly CW. Comparison of the incidence of Listeria on equipment versus environmental sites within dairy processing plants. Int J Food Microbiol. 1995;26:375–384. - PubMed
-
- Tompkin RB. Control of Listeria monocytogenes in the food-processing environment. J Food Prot. 2002;65:709–725. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

