High ACSL5 transcript levels associate with systemic lupus erythematosus and apoptosis in Jurkat T lymphocytes and peripheral blood cells
- PMID: 22163040
- PMCID: PMC3232234
- DOI: 10.1371/journal.pone.0028591
High ACSL5 transcript levels associate with systemic lupus erythematosus and apoptosis in Jurkat T lymphocytes and peripheral blood cells
Abstract
Background: Systemic lupus erythematosus (SLE) is a prototypical autoimmune disease in which increased apoptosis and decreased apoptotic cells removal has been described as most relevant in the pathogenesis. Long-chain acyl-coenzyme A synthetases (ACSLs) have been involved in the immunological dysfunction of mouse models of lupus-like autoimmunity and apoptosis in different in vitro cell systems. The aim of this work was to assess among the ACSL isoforms the involvement of ACSL2, ACSL4 and ACSL5 in SLE pathogenesis.
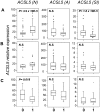
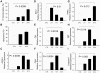
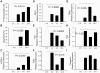
Findings: With this end, we determined the ACSL2, ACSL4 and ACSL5 transcript levels in peripheral blood mononuclear cells (PBMCs) of 45 SLE patients and 49 healthy controls by quantitative real time-PCR (q-PCR). We found that patients with SLE had higher ACSL5 transcript levels than healthy controls [median (range), healthy controls = 16.5 (12.3-18.0) vs. SLE = 26.5 (17.8-41.7), P = 3.9×10 E-5] but no differences were found for ACSL2 and ACSL4. In in vitro experiments, ACSL5 mRNA expression was greatly increased when inducing apoptosis in Jurkat T cells and PBMCs by Phorbol-Myristate-Acetate plus Ionomycin (PMA+Io). On the other hand, short interference RNA (siRNA)-mediated silencing of ACSL5 decreased induced apoptosis in Jurkat T cells up to the control levels as well as decreased mRNA expression of FAS, FASLG and TNF.
Conclusions: These findings indicate that ACSL5 may play a role in the apoptosis that takes place in SLE. Our results point to ACSL5 as a potential novel functional marker of pathogenesis and a possible therapeutic target in SLE.
Conflict of interest statement
Figures

References
-
- D'Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet. 2007;369:587–96. - PubMed
-
- Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, et al. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241–50. - PubMed
-
- Vermes I, Haanen C, Richel DJ, Schaafsma MR, Kalsbeek-Batenburg E, et al. Apoptosis and secondary necrosis of lymphocytes in culture. Acta Haematol. 1997;98:8–13. - PubMed
-
- Dhir V, Singh AP, Aggarwal A, Naik S, Misra R. Increased T-lymphocyte apoptosis in lupus correlates with disease activity and may be responsible for reduced T-cell frequency: a cross-sectional and longitudinal study. Lupus. 2009;18:785–91. - PubMed
-
- Mevorach D. Systemic lupus erythematosus and apoptosis: a question of balance. Clin Rev Immunol. 2003;25:49–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

