Genetic knockdown and pharmacological inhibition of parasite multidrug resistance transporters disrupts egg production in Schistosoma mansoni
- PMID: 22163059
- PMCID: PMC3232217
- DOI: 10.1371/journal.pntd.0001425
Genetic knockdown and pharmacological inhibition of parasite multidrug resistance transporters disrupts egg production in Schistosoma mansoni
Abstract
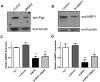
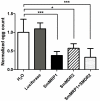
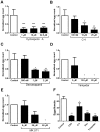
P-glycoprotein (Pgp) and multidrug resistance-associated proteins (MRPs) are ATP-dependent transporters involved in efflux of toxins and xenobiotics from cells. When overexpressed, these transporters can mediate multidrug resistance (MDR) in mammalian cells, and changes in Pgp expression and sequence are associated with drug resistance in helminths. In addition to the role they play in drug efflux, MDR transporters are essential components of normal cellular physiology, and targeting them may prove a useful strategy for development of new therapeutics or of compounds that enhance the efficacy of current anthelmintics. We previously showed that expression of Schistosoma mansoni MDR transporters increases in response to praziquantel (PZQ), the current drug of choice against schistosomiasis, and that reduced PZQ sensitivity correlates with higher levels of these parasite transporters. We have also shown that PZQ inhibits transport by SMDR2, a Pgp orthologue from S. mansoni, and that PZQ is a likely substrate of SMDR2. Here, we examine the physiological roles of SMDR2 and SmMRP1 (the S. mansoni orthologue of MRP1) in S. mansoni adults, using RNAi to knock down expression, and pharmacological agents to inhibit transporter function. We find that both types of treatments disrupt parasite egg deposition by worms in culture. Furthermore, administration of different MDR inhibitors to S. mansoni-infected mice results in a reduction in egg burden in host liver. These schistosome MDR transporters therefore appear to play essential roles in parasite egg production, and can be targeted genetically and pharmacologically. Since eggs are responsible for the major pathophysiological consequences of schistosomiasis, and since they are also the agents for transmission of the disease, these results suggest a potential strategy for reducing disease pathology and spread.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

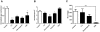
Similar articles
-
Schistosoma mansoni express higher levels of multidrug resistance-associated protein 1 (SmMRP1) in juvenile worms and in response to praziquantel.Mol Biochem Parasitol. 2010 Sep;173(1):25-31. doi: 10.1016/j.molbiopara.2010.05.003. Epub 2010 May 12. Mol Biochem Parasitol. 2010. PMID: 20470831 Free PMC article.
-
Schistosoma mansoni P-glycoprotein levels increase in response to praziquantel exposure and correlate with reduced praziquantel susceptibility.Mol Biochem Parasitol. 2009 Sep;167(1):54-9. doi: 10.1016/j.molbiopara.2009.04.007. Epub 2009 May 3. Mol Biochem Parasitol. 2009. PMID: 19406169 Free PMC article.
-
Inhibition or knockdown of ABC transporters enhances susceptibility of adult and juvenile schistosomes to Praziquantel.PLoS Negl Trop Dis. 2014 Oct 16;8(10):e3265. doi: 10.1371/journal.pntd.0003265. eCollection 2014 Oct. PLoS Negl Trop Dis. 2014. PMID: 25330312 Free PMC article.
-
Schistosome ABC multidrug transporters: From pharmacology to physiology.Int J Parasitol Drugs Drug Resist. 2014 Sep 26;4(3):301-9. doi: 10.1016/j.ijpddr.2014.09.007. eCollection 2014 Dec. Int J Parasitol Drugs Drug Resist. 2014. PMID: 25516841 Free PMC article. Review.
-
ABC multidrug transporters in schistosomes and other parasitic flatworms.Parasitol Int. 2013 Dec;62(6):647-53. doi: 10.1016/j.parint.2013.02.006. Epub 2013 Mar 6. Parasitol Int. 2013. PMID: 23474413 Free PMC article. Review.
Cited by
-
Applications of RNA Interference in Schistosomiasis: Gene Function Identification and Development of New Therapies.ISRN Parasitol. 2012 Dec 31;2013:247036. doi: 10.5402/2013/247036. eCollection 2013. ISRN Parasitol. 2012. PMID: 27335847 Free PMC article. Review.
-
shRNA-mediated silencing of sorcin increases drug chemosensitivity in myeloma KM3/DDP and U266/ADM cell lines.Int J Clin Exp Pathol. 2015 Mar 1;8(3):2300-10. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26045737 Free PMC article.
-
Praziquantel treatment decreases Schistosoma mansoni genetic diversity in experimental infections.PLoS Negl Trop Dis. 2013 Dec 19;7(12):e2596. doi: 10.1371/journal.pntd.0002596. eCollection 2013. PLoS Negl Trop Dis. 2013. PMID: 24367712 Free PMC article.
-
Application of RNAi to Genomic Drug Target Validation in Schistosomes.PLoS Negl Trop Dis. 2015 May 20;9(5):e0003801. doi: 10.1371/journal.pntd.0003801. eCollection 2015 May. PLoS Negl Trop Dis. 2015. PMID: 25992548 Free PMC article.
-
Evidence for Novel Pharmacological Sensitivities of Transient Receptor Potential (TRP) Channels in Schistosoma mansoni.PLoS Negl Trop Dis. 2015 Dec 11;9(12):e0004295. doi: 10.1371/journal.pntd.0004295. eCollection 2015 Dec. PLoS Negl Trop Dis. 2015. PMID: 26655809 Free PMC article.
References
-
- King CH, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illn. 2008;4:65–79. - PubMed
-
- van der Werf MJ, de Vlas SJ, Brooker S, Looman CW, Nagelkerke NJ, et al. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86:125–139. - PubMed
-
- Kusel JR, McVeigh P, Thornhill JA. The schistosome excretory system: a key to regulation of metabolism, drug excretion and host interaction. Trends Parasitol. 2009;25:353–358. - PubMed
-
- Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM. P-glycoprotein: from genomics to mechanism. Oncogene. 2003;22:7468–7485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

