Directed evaluation of enterotoxigenic Escherichia coli autotransporter proteins as putative vaccine candidates
- PMID: 22163060
- PMCID: PMC3232201
- DOI: 10.1371/journal.pntd.0001428
Directed evaluation of enterotoxigenic Escherichia coli autotransporter proteins as putative vaccine candidates
Abstract
Background: Enterotoxigenic Escherichia coli (ETEC) is a major diarrheal pathogen in developing countries, where it accounts for millions of infections and hundreds of thousands of deaths annually. While vaccine development to prevent diarrheal illness due to ETEC is feasible, extensive effort is needed to identify conserved antigenic targets. Pathogenic Escherichia coli, including ETEC, use the autotransporter (AT) secretion mechanism to export virulence factors. AT proteins are comprised of a highly conserved carboxy terminal outer membrane beta barrel and a surface-exposed amino terminal passenger domain. Recent immunoproteomic studies suggesting that multiple autotransporter passenger domains are recognized during ETEC infection prompted the present studies.
Methodology: Available ETEC genomes were examined to identify AT coding sequences present in pathogenic isolates, but not in the commensal E. coli HS strain. Passenger domains of the corresponding autotransporters were cloned and expressed as recombinant antigens, and the immune response to these proteins was then examined using convalescent sera from patients and experimentally infected mice.
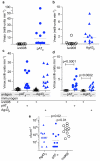
Principal findings: Potential AT genes shared by ETEC strains, but absent in the E. coli commensal HS strain were identified. Recombinant passenger domains derived from autotransporters, including Ag43 and an AT designated pAT, were recognized by antibodies from mice following intestinal challenge with H10407, and both Ag43 and pAT were identified on the surface of ETEC by flow cytometry. Likewise, convalescent sera from patients with ETEC diarrhea recognized Ag43 and pAT, suggesting that these proteins are expressed during both experimental and naturally occurring ETEC infections and that they are immunogenic. Vaccination of mice with recombinant passenger domains from either pAT or Ag43 afforded protection against intestinal colonization with ETEC.
Conclusions: Passenger domains of conserved autotransporter proteins could contribute to protective immune responses that develop following infection with ETEC, and these antigens consequently represent potential targets to explore in vaccine development.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Immunization with the MipA, Skp, or ETEC_2479 Antigens Confers Protection against Enterotoxigenic E. coli Strains Expressing Different Colonization Factors in a Mouse Pulmonary Challenge Model.Front Cell Infect Microbiol. 2016 Dec 12;6:181. doi: 10.3389/fcimb.2016.00181. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 28018863 Free PMC article.
-
Protective Enterotoxigenic Escherichia coli Antigens in a Murine Intranasal Challenge Model.PLoS Negl Trop Dis. 2015 Aug 5;9(8):e0003924. doi: 10.1371/journal.pntd.0003924. eCollection 2015. PLoS Negl Trop Dis. 2015. PMID: 26244636 Free PMC article.
-
Preclinical Characterization of Immunogenicity and Efficacy against Diarrhea from MecVax, a Multivalent Enterotoxigenic E. coli Vaccine Candidate.Infect Immun. 2021 Jun 16;89(7):e0010621. doi: 10.1128/IAI.00106-21. Epub 2021 Jun 16. Infect Immun. 2021. PMID: 33875477 Free PMC article.
-
Strategies to overexpress enterotoxigenic Escherichia coli (ETEC) colonization factors for the construction of oral whole-cell inactivated ETEC vaccine candidates.Appl Microbiol Biotechnol. 2012 Mar;93(6):2291-300. doi: 10.1007/s00253-012-3930-6. Epub 2012 Feb 16. Appl Microbiol Biotechnol. 2012. PMID: 22350259 Review.
-
Review on pathogenicity mechanism of enterotoxigenic Escherichia coli and vaccines against it.Microb Pathog. 2018 Apr;117:162-169. doi: 10.1016/j.micpath.2018.02.032. Epub 2018 Feb 21. Microb Pathog. 2018. PMID: 29474827 Review.
Cited by
-
Human Mucosal IgA Immune Responses against Enterotoxigenic Escherichia coli.Pathogens. 2020 Aug 29;9(9):714. doi: 10.3390/pathogens9090714. Pathogens. 2020. PMID: 32872549 Free PMC article. Review.
-
The Diversity of Escherichia coli Pathotypes and Vaccination Strategies against This Versatile Bacterial Pathogen.Microorganisms. 2023 Jan 30;11(2):344. doi: 10.3390/microorganisms11020344. Microorganisms. 2023. PMID: 36838308 Free PMC article. Review.
-
Surface proteome mining for identification of potential vaccine candidates against Campylobacter jejuni: an in silico approach.Funct Integr Genomics. 2017 Jan;17(1):27-37. doi: 10.1007/s10142-016-0530-z. Epub 2016 Oct 24. Funct Integr Genomics. 2017. PMID: 27778110
-
Enterotoxigenic Escherichia coli Infections.Curr Infect Dis Rep. 2019 Mar 4;21(3):9. doi: 10.1007/s11908-019-0665-x. Curr Infect Dis Rep. 2019. PMID: 30830466 Free PMC article. Review.
-
Cooperative role of antibodies against heat-labile toxin and the EtpA Adhesin in preventing toxin delivery and intestinal colonization by enterotoxigenic Escherichia coli.Clin Vaccine Immunol. 2012 Oct;19(10):1603-8. doi: 10.1128/CVI.00351-12. Epub 2012 Aug 8. Clin Vaccine Immunol. 2012. PMID: 22875600 Free PMC article.
References
-
- WHO. Future directions for research on enterotoxigenic Escherichia coli vaccines for developing countries. Wkly Epidemiol Rec. 2006;81:97–104. - PubMed
-
- Al-Abri SS, Beeching NJ, Nye FJ. Traveller's diarrhoea. Lancet Infect Dis. 2005;5:349–360. - PubMed
-
- Enterotoxigenic Escherichia coli: advances in technical and laboratory aspects of research and development of vaccines. Wkly Epidemiol Rec. 2008;83:92–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

