Chemical synthesis and functionalization of clickable glycosylphosphatidylinositol anchors
- PMID: 22163072
- PMCID: PMC3233219
- DOI: 10.1039/C1SC00440A
Chemical synthesis and functionalization of clickable glycosylphosphatidylinositol anchors
Abstract
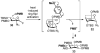
Glycosylphosphatidylinositol (GPI) anchorage is a common posttranslational modification of eukaryotic proteins. Chemical synthesis of structurally defined GPIs and GPI derivatives is a necessary step toward understanding the properties and functions of these molecules in biological systems. In this work, the synthesis of several functionalized GPI anchors was accomplished using the para-methoxybenzyl (PMB) group for permanent hydroxyl protection, which allowed the incorporation of functionalities that are incompatible with permanent protecting groups traditionally used in carbohydrate synthesis. A flexible convergent-divergent assembly strategy enabled efficient access to a diverse set of target structures, including "clickable" Alkynyl-GPIs 1 and 2 and Azido-GPI 3. For global deprotection, a one-pot reaction was employed to afford the target GPIs in excellent yields (85-97%). Fully deprotected clickable GPIs 2 and 3 were readily conjugated to imaging and affinity probes via Cu(I)-catalyzed and Cu-free strain-promoted [3+2] cycloaddition, respectively, resulting in GPI-Fluor 4 and GPI-Biotin 5.
Figures














References
Grants and funding
LinkOut - more resources
Full Text Sources

