Redistribution of DAT/α-synuclein complexes visualized by "in situ" proximity ligation assay in transgenic mice modelling early Parkinson's disease
- PMID: 22163275
- PMCID: PMC3233557
- DOI: 10.1371/journal.pone.0027959
Redistribution of DAT/α-synuclein complexes visualized by "in situ" proximity ligation assay in transgenic mice modelling early Parkinson's disease
Erratum in
- PLoS One. 2012;7(1). doi:10.1371/annotation/58679d17-c619-4f13-b11f-146dacfe7e92. Grazia, Maria [corrected to Spillantini, Maria Grazia]
Abstract
Alpha-synuclein, the major component of Lewy bodies, is thought to play a central role in the onset of synaptic dysfunctions in Parkinson's disease (PD). In particular, α-synuclein may affect dopaminergic neuron function as it interacts with a key protein modulating dopamine (DA) content at the synapse: the DA transporter (DAT). Indeed, recent evidence from our "in vitro" studies showed that α-synuclein aggregation decreases the expression and membrane trafficking of the DAT as the DAT is retained into α-synuclein-immunopositive inclusions. This notwithstanding, "in vivo" studies on PD animal models investigating whether DAT distribution is altered by the pathological overexpression and aggregation of α-synuclein are missing. By using the proximity ligation assay, a technique which allows the "in situ" visualization of protein-protein interactions, we studied the occurrence of alterations in the distribution of DAT/α-synuclein complexes in the SYN120 transgenic mouse model, showing insoluble α-synuclein aggregates into dopaminergic neurons of the nigrostriatal system, reduced striatal DA levels and an altered distribution of synaptic proteins in the striatum. We found that DAT/α-synuclein complexes were markedly redistributed in the striatum and substantia nigra of SYN120 mice. These alterations were accompanied by a significant increase of DAT striatal levels in transgenic animals when compared to wild type littermates. Our data indicate that, in the early pathogenesis of PD, α-synuclein acts as a fine modulator of the dopaminergic synapse by regulating the subcellular distribution of key proteins such as the DAT.
Conflict of interest statement
Figures

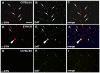
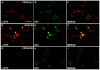

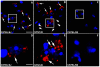
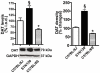

References
-
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. - PubMed
-
- Cookson MR. The biochemistry of Parkinson's disease. Annu. Rev. Biochem. 2005;74:29–52. - PubMed
-
- Uversky VN. Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J Neurochem. 2007;103:17–37. - PubMed
-
- Kovacs GG, Milenkovic IJ, Preusser M, Budka H. Nigral burden of alpha-synuclein correlates with striatal dopamine deficit. Mov Disord. 2008;23:1608–1612. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

