Enzyme immobilization strategies and electropolymerization conditions to control sensitivity and selectivity parameters of a polymer-enzyme composite glucose biosensor
- PMID: 22163559
- PMCID: PMC3231131
- DOI: 10.3390/s100706439
Enzyme immobilization strategies and electropolymerization conditions to control sensitivity and selectivity parameters of a polymer-enzyme composite glucose biosensor
Abstract
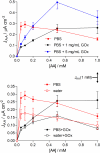
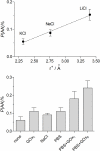
In an ongoing programme to develop characterization strategies relevant to biosensors for in-vivo monitoring, glucose biosensors were fabricated by immobilizing the enzyme glucose oxidase (GOx) on 125 μm diameter Pt cylinder wire electrodes (Pt(C)), using three different methods: before, after or during the amperometric electrosynthesis of poly(ortho-phenylenediamine), PoPD, which also served as a permselective membrane. These electrodes were calibrated with H(2)O(2) (the biosensor enzyme signal molecule), glucose, and the archetypal interference compound ascorbic acid (AA) to determine the relevant polymer permeabilities and the apparent Michaelis-Menten parameters for glucose. A number of selectivity parameters were used to identify the most successful design in terms of the balance between substrate sensitivity and interference blocking. For biosensors electrosynthesized in neutral buffer under the present conditions, entrapment of the GOx within the PoPD layer produced the design (Pt(C)/PoPD-GOx) with the highest linear sensitivity to glucose (5.0 ± 0.4 μA cm(-2) mM(-1)), good linear range (K(M) = 16 ± 2 mM) and response time (< 2 s), and the greatest AA blocking (99.8% for 1 mM AA). Further optimization showed that fabrication of Pt(C)/PoPD-GOx in the absence of added background electrolyte (i.e., electropolymerization in unbuffered enzyme-monomer solution) enhanced glucose selectivity 3-fold for this one-pot fabrication protocol which provided AA-rejection levels at least equal to recent multi-step polymer bilayer biosensor designs. Interestingly, the presence of enzyme protein in the polymer layer had opposite effects on permselectivity for low and high concentrations of AA, emphasizing the value of studying the concentration dependence of interference effects which is rarely reported in the literature.
Keywords: amperometry; ascorbic acid interference; brain monitoring; enzyme-modified electrode; hydrogen peroxide; polyphenylenediamine.
Figures

Similar articles
-
Glucose biosensor based on immobilization of glucose oxidase in poly(o-aminophenol) film on polypyrrole-Pt nanocomposite modified glassy carbon electrode.Biosens Bioelectron. 2007 Jun 15;22(12):2898-905. doi: 10.1016/j.bios.2006.12.004. Epub 2007 Jan 9. Biosens Bioelectron. 2007. PMID: 17215117
-
Amperometric glucose biosensor based on electroconductive hydrogels.Talanta. 2013 Jan 15;103:228-35. doi: 10.1016/j.talanta.2012.10.037. Epub 2012 Nov 1. Talanta. 2013. PMID: 23200382
-
Contributions by a novel edge effect to the permselectivity of an electrosynthesized polymer for microbiosensor applications.Anal Chem. 2009 May 15;81(10):3911-8. doi: 10.1021/ac900162c. Anal Chem. 2009. PMID: 19371060
-
Enzyme-based amperometric biosensors: 60 years later … Quo Vadis?Anal Chim Acta. 2022 Nov 22;1234:340517. doi: 10.1016/j.aca.2022.340517. Epub 2022 Oct 14. Anal Chim Acta. 2022. PMID: 36328722 Review.
-
Application of Chemometrics in Biosensing: A Review.Biosensors (Basel). 2020 Aug 17;10(8):100. doi: 10.3390/bios10080100. Biosensors (Basel). 2020. PMID: 32824611 Free PMC article. Review.
Cited by
-
Development of Screen-Printed Electrode Biosensor for Rapid Determination of Triglyceride Content in Coconut Milk.Int J Food Sci. 2020 May 6;2020:1696201. doi: 10.1155/2020/1696201. eCollection 2020. Int J Food Sci. 2020. PMID: 32455128 Free PMC article.
-
Further in-vitro characterization of an implantable biosensor for ethanol monitoring in the brain.Sensors (Basel). 2013 Jul 23;13(7):9522-35. doi: 10.3390/s130709522. Sensors (Basel). 2013. PMID: 23881145 Free PMC article.
-
The Presence of Polysaccharides, Glycerol, and Polyethyleneimine in Hydrogel Enhances the Performance of the Glucose Biosensor.Biosensors (Basel). 2019 Jul 30;9(3):95. doi: 10.3390/bios9030095. Biosensors (Basel). 2019. PMID: 31366026 Free PMC article.
-
Enzyme Biosensors for Biomedical Applications: Strategies for Safeguarding Analytical Performances in Biological Fluids.Sensors (Basel). 2016 May 30;16(6):780. doi: 10.3390/s16060780. Sensors (Basel). 2016. PMID: 27249001 Free PMC article. Review.
-
Low-Temperature Storage Improves the Over-Time Stability of Implantable Glucose and Lactate Biosensors.Sensors (Basel). 2019 Jan 21;19(2):422. doi: 10.3390/s19020422. Sensors (Basel). 2019. PMID: 30669626 Free PMC article.
References
-
- Hirst ER, Yuan YJ, Xu WL, Bronlund JE. Bond-rupture immunosensors—A review. Biosens. Bioelectron. 2008;23:1759–1768. - PubMed
-
- Wanekaya AK, Chen W, Mulchandani A. Recent biosensing developments in environmental security. J. Environ. Monit. 2008;10:703–712. - PubMed
-
- Sadik OA, Aluoch AO, Zhou AL. Status of biomolecular recognition using electrochemical techniques. Biosens. Bioelectron. 2009;24:2749–2765. - PubMed
-
- Sozer N, Kokini JL. Nanotechnology and its applications in the food sector. Trends Biotechnol. 2009;27:82–89. - PubMed
-
- Saito H, Nakazato T, Ishii N, Kudo H, Otsuka K, Endo H, Mitsubayashi K. An optical flow injection analysis system for measurement of glucose in tomato. Eur. Food Res. Technol. 2008;227:473–478.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

