Hedonic judgments of chemical compounds are correlated with molecular size
- PMID: 22163815
- PMCID: PMC3231300
- DOI: 10.3390/s110403667
Hedonic judgments of chemical compounds are correlated with molecular size
Abstract
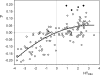
Different psychophysical works have reported that, when a wide range of odors is assessed, the hedonic dimension is the most salient. Hence, pleasantness is the most basic attribute of odor perception. Recent studies suggest that the molecular size of a given odorant is positively correlated with its hedonic character. This correlation was confirmed in the present study, but further basic molecular features affecting pleasantness were identified by means of multiple linear regression for the compounds contained in five chemical sets. For three of them, hedonic judgments are available in the literature. For a further two chemical sets, hedonic scores were estimated from odor character descriptions based on numerical profiles. Generally speaking, fairly similar equations were obtained for the prediction of hedonic judgments in the five chemical sets, with R(2) values ranging from 0.46 to 0.71. The results suggest that larger molecules containing oxygen are more likely to be perceived as pleasant, while the opposite applies to carboxylic acids and sulfur compounds.
Keywords: VOC; hedonic valence; numerical odor profile; odor character descriptor; olfaction; pleasantness.
Figures

References
-
- Pearce TC, Schiffman SS, Nagle HT, Gardner JW. Handbook of Machine Olfaction: Electronic Nose Technology. Wiley-VCH; Weinheim, Germany: 2003.
-
- Zarzo M. The sense of smell: Molecular basis of odorant recognition. Biol. Rev. 2007;82:455–479. - PubMed
-
- Linnaeus C. Amoenitates Academicae. Vol. 3. Lars Salvius; Stockholm, Sweden: 1756. Odores medicamentorum; pp. 183–201.
-
- von Haller A. Elementa Physiologiae. Vol. 5. Grasset; Lausanne, Switzerland: 1763. Classes odorum; pp. 162–168. Book 14, Section 2,
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

