Development of an integrated microfluidic perfusion cell culture system for real-time microscopic observation of biological cells
- PMID: 22164082
- PMCID: PMC3231477
- DOI: 10.3390/s110908395
Development of an integrated microfluidic perfusion cell culture system for real-time microscopic observation of biological cells
Abstract
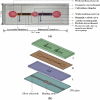
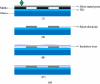
This study reports an integrated microfluidic perfusion cell culture system consisting of a microfluidic cell culture chip, and an indium tin oxide (ITO) glass-based microheater chip for micro-scale perfusion cell culture, and its real-time microscopic observation. The system features in maintaining both uniform, and stable chemical or thermal environments, and providing a backflow-free medium pumping, and a precise thermal control functions. In this work, the performance of the medium pumping scheme, and the ITO glass microheater were experimentally evaluated. Results show that the medium delivery mechanism was able to provide pumping rates ranging from 15.4 to 120.0 μL·min(-1). In addition, numerical simulation and experimental evaluation were conducted to verify that the ITO glass microheater was capable of providing a spatially uniform thermal environment, and precise temperature control with a mild variation of ±0.3 °C. Furthermore, a perfusion cell culture was successfully demonstrated, showing the cultured cells were kept at high cell viability of 95 ± 2%. In the process, the cultured chondrocytes can be clearly visualized microscopically. As a whole, the proposed cell culture system has paved an alternative route to carry out real-time microscopic observation of biological cells in a simple, user-friendly, and low cost manner.
Keywords: ITO glass; cell culture; microfluidics; microheaters; micropumps.
Figures






References
-
- Xu X, Urban JPG, Browning JA, Tirlapur U, Wilkins RJ, Wu MH, Cui Z, Cui ZF. Influences of buffer systems on chondrocyte growth during long-term culture in alginate. Osteoarthritis Cartilage. 2007;15:396–402. - PubMed
-
- Wu MH, Urban JPG, Cui ZF, Cui Z, Xu X. Effect of extracellular pH on matrix synthesis by chondrocytes in 3D agarose gel. Biotechnol. Prog. 2007;23:430–434. - PubMed
-
- Wu MH, Huang SB, Cui ZF, Cui Z, Lee GB. Development of perfusion-based micro 3-D cell culture platform and its application for high throughput drug testing. Sens. Actuat. B. 2008;129:231–240.
-
- Cui ZF, Xu X, Trainor N, Triffitt JT, Urban JP, Tirlapur UK. Application of multiple parallel perfused microbioreactors and three-dimensional stem cell culture for toxicity testing. Toxicol. In Vitro. 2007;21:1318–1324. - PubMed
-
- Wu MH, Kuo CY. Application of high throughput perfusion micro 3-D cell culture platform for the precise study of cellular responses to extracellular conditions-effect of serum concentrations on the physiology of articular chondrocytes. Biomed. Microdevices. 2011;13:131–141. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

