Lipids, curvature, and nano-medicine
- PMID: 22164124
- PMCID: PMC3229985
- DOI: 10.1002/ejlt.201100050
Lipids, curvature, and nano-medicine
Abstract
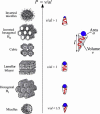
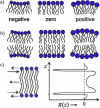
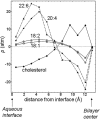
The physical properties of the lamellar lipid-bilayer component of biological membranes are controlled by a host of thermodynamic forces leading to overall tensionless bilayers with a conspicuous lateral pressure profile and build-in curvature-stress instabilities that may be released locally or globally in terms of morphological changes. In particular, the average molecular shape and the propensity of the different lipid and protein species for forming non-lamellar and curved structures are a source of structural transitions and control of biological function. The effects of different lipids, sterols, and proteins on membrane structure are discussed and it is shown how one can take advantage of the curvature-stress modulations brought about by specific molecular agents, such as fatty acids, lysolipids, and other amphiphilic solutes, to construct intelligent drug-delivery systems that function by enzymatic triggering via curvature.Practical applications: The simple concept of lipid molecular shape and how it impacts on the structure of lipid aggregates, in particular the curvature and curvature stress in lipid bilayers and liposomes, can be exploited to construct liposome-based drug-delivery systems, e.g., for use as nano-medicine in cancer therapy. Non-lamellar-forming lysolipids and fatty acids, some of which may be designed to be prodrugs, can be created by phospholipase action in diseased tissues thereby providing for targeted drug release and proliferation of molecular entities with conical shape that break down the permeability barrier of the target cells and may hence enhance efficacy.
Figures

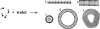





Similar articles
-
Is the fluid mosaic (and the accompanying raft hypothesis) a suitable model to describe fundamental features of biological membranes? What may be missing?Front Plant Sci. 2013 Nov 13;4:457. doi: 10.3389/fpls.2013.00457. Front Plant Sci. 2013. PMID: 24312108 Free PMC article. Review.
-
Lipids, curvature stress, and the action of lipid prodrugs: free fatty acids and lysolipid enhancement of drug transport across liposomal membranes.Biochimie. 2012 Jan;94(1):2-10. doi: 10.1016/j.biochi.2011.07.029. Epub 2011 Aug 3. Biochimie. 2012. PMID: 21839138
-
Membrane-perturbing effect of fatty acids and lysolipids.Prog Lipid Res. 2013 Jan;52(1):130-40. doi: 10.1016/j.plipres.2012.09.002. Epub 2012 Oct 29. Prog Lipid Res. 2013. PMID: 23117036 Review.
-
Effect of fatty acids on the permeability barrier of model and biological membranes.Chem Phys Lipids. 2016 Oct;200:139-146. doi: 10.1016/j.chemphyslip.2016.10.001. Epub 2016 Oct 8. Chem Phys Lipids. 2016. PMID: 27725161
-
Enzymatic release of antitumor ether lipids by specific phospholipase A2 activation of liposome-forming prodrugs.J Med Chem. 2004 Mar 25;47(7):1694-703. doi: 10.1021/jm031029r. J Med Chem. 2004. PMID: 15027860
Cited by
-
Molecular model for the solubilization of membranes into nanodisks by styrene maleic Acid copolymers.Biophys J. 2015 Jan 20;108(2):279-90. doi: 10.1016/j.bpj.2014.11.3464. Biophys J. 2015. PMID: 25606677 Free PMC article.
-
Krill Oil-Incorporated Liposomes As An Effective Nanovehicle To Ameliorate The Inflammatory Responses Of DSS-Induced Colitis.Int J Nanomedicine. 2019 Nov 6;14:8305-8320. doi: 10.2147/IJN.S220053. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31806959 Free PMC article.
-
Self-assembly of size-controlled liposomes on DNA nanotemplates.Nat Chem. 2016 May;8(5):476-83. doi: 10.1038/nchem.2472. Epub 2016 Mar 21. Nat Chem. 2016. PMID: 27102682 Free PMC article.
-
What We Need to Know about Liposomes as Drug Nanocarriers: An Updated Review.Adv Pharm Bull. 2023 Jan;13(1):7-23. doi: 10.34172/apb.2023.009. Epub 2022 Apr 4. Adv Pharm Bull. 2023. PMID: 36721822 Free PMC article. Review.
-
Synthetic Approaches for Nucleic Acid Delivery: Choosing the Right Carriers.Life (Basel). 2019 Jul 9;9(3):59. doi: 10.3390/life9030059. Life (Basel). 2019. PMID: 31324016 Free PMC article. Review.
References
-
- Bagatolli LA, Ipsen JH, Simonsen AC, Mouritsen OG. An outlook on organization of lipids in membranes: Searching for a realistic connection with the organization of biological membranes. Prog. Lipid Res. 2010;49:378–389. - PubMed
-
- Sackmann E. In: Handbook of Biological Physics, Structure and Dynamics of Membranes. Lipowsky R, Sackmann E, editors. 1A. Amsterdam, Holland: Elsevier; 1995. pp. 1–63.
-
- Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. - PubMed
-
- Israelachvili JN. Refinement of the fluid-mosaic model of membrane structure. Biochim. Biophys. Acta. 1977;469:221–225. - PubMed
LinkOut - more resources
Full Text Sources
