Concentration dependent ion selectivity in VDAC: a molecular dynamics simulation study
- PMID: 22164223
- PMCID: PMC3229507
- DOI: 10.1371/journal.pone.0027994
Concentration dependent ion selectivity in VDAC: a molecular dynamics simulation study
Abstract
The voltage-dependent anion channel (VDAC) forms the major pore in the outer mitochondrial membrane. Its high conducting open state features a moderate anion selectivity. There is some evidence indicating that the electrophysiological properties of VDAC vary with the salt concentration. Using a theoretical approach the molecular basis for this concentration dependence was investigated. Molecular dynamics simulations and continuum electrostatic calculations performed on the mouse VDAC1 isoform clearly demonstrate that the distribution of fixed charges in the channel creates an electric field, which determines the anion preference of VDAC at low salt concentration. Increasing the salt concentration in the bulk results in a higher concentration of ions in the VDAC wide pore. This event induces a large electrostatic screening of the charged residues promoting a less anion selective channel. Residues that are responsible for the electrostatic pattern of the channel were identified using the molecular dynamics trajectories. Some of these residues are found to be conserved suggesting that ion permeation between different VDAC species occurs through a common mechanism. This inference is buttressed by electrophysiological experiments performed on bean VDAC32 protein akin to mouse VDAC.
Conflict of interest statement
Figures
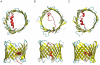
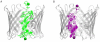
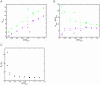


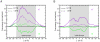

Similar articles
-
Molecular origin of VDAC selectivity towards inorganic ions: a combined molecular and Brownian dynamics study.Biochim Biophys Acta. 2013 Apr;1828(4):1284-92. doi: 10.1016/j.bbamem.2012.12.018. Epub 2013 Jan 9. Biochim Biophys Acta. 2013. PMID: 23313453
-
Lipid composition and salt concentration as regulatory factors of the anion selectivity of VDAC studied by coarse-grained molecular dynamics simulations.Chem Phys Lipids. 2019 May;220:66-76. doi: 10.1016/j.chemphyslip.2018.11.002. Epub 2018 Nov 15. Chem Phys Lipids. 2019. PMID: 30448398
-
Modulation of the mitochondrial voltage-dependent anion channel (VDAC) by hydrogen peroxide and its recovery by curcumin.Eur Biophys J. 2020 Oct;49(7):661-672. doi: 10.1007/s00249-020-01469-2. Epub 2020 Oct 24. Eur Biophys J. 2020. PMID: 33098437
-
The VDAC channel: Molecular basis for selectivity.Biochim Biophys Acta. 2016 Oct;1863(10):2498-502. doi: 10.1016/j.bbamcr.2016.01.019. Epub 2016 Jan 27. Biochim Biophys Acta. 2016. PMID: 26826035 Review.
-
Plant VDAC: facts and speculations.Biochim Biophys Acta. 2012 Jun;1818(6):1486-501. doi: 10.1016/j.bbamem.2011.11.028. Epub 2011 Dec 3. Biochim Biophys Acta. 2012. PMID: 22155681 Review.
Cited by
-
Dual mechanism of ion permeation through VDAC revealed with inorganic phosphate ions and phosphate metabolites.PLoS One. 2015 Apr 10;10(4):e0121746. doi: 10.1371/journal.pone.0121746. eCollection 2015. PLoS One. 2015. PMID: 25860993 Free PMC article.
-
The Open State Selectivity of the Bean Seed VDAC Depends on Stigmasterol and Ion Concentration.Int J Mol Sci. 2021 Mar 16;22(6):3034. doi: 10.3390/ijms22063034. Int J Mol Sci. 2021. PMID: 33809742 Free PMC article.
-
Flexibility of the N-terminal mVDAC1 segment controls the channel's gating behavior.PLoS One. 2012;7(10):e47938. doi: 10.1371/journal.pone.0047938. Epub 2012 Oct 23. PLoS One. 2012. PMID: 23110136 Free PMC article.
-
Charged residues distribution modulates selectivity of the open state of human isoforms of the voltage dependent anion-selective channel.PLoS One. 2014 Aug 1;9(8):e103879. doi: 10.1371/journal.pone.0103879. eCollection 2014. PLoS One. 2014. PMID: 25084457 Free PMC article.
-
Computational investigation of cholesterol binding sites on mitochondrial VDAC.J Phys Chem B. 2014 Aug 21;118(33):9852-60. doi: 10.1021/jp504516a. Epub 2014 Aug 11. J Phys Chem B. 2014. PMID: 25080204 Free PMC article.
References
-
- Colombini M. Structure and mode of action of a voltage dependent anion-selective channel (VDAC) located in the outer mitochondrial membrane. Ann N Y Acad Sci. 1980;341:552–563. - PubMed
-
- Shoshan-Barmatz V, De Pinto V, Zweckstetter M, Raviv Z, Keinan N, et al. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol Aspects Med. 2010;31:227–285. S0098-2997(10)00021-X [pii];10.1016/j.mam.2010.03.002 [doi] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

