Glycosylation of erythrocyte spectrin and its modification in visceral leishmaniasis
- PMID: 22164239
- PMCID: PMC3229537
- DOI: 10.1371/journal.pone.0028169
Glycosylation of erythrocyte spectrin and its modification in visceral leishmaniasis
Abstract
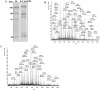
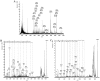
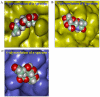
Using a lectin, Achatinin-H, having preferential specificity for glycoproteins with terminal 9-O-acetyl sialic acid derivatives linked in α2-6 linkages to subterminal N-acetylgalactosamine, eight distinct disease-associated 9-O-acetylated sialoglycoproteins was purified from erythrocytes of visceral leishmaniaisis (VL) patients (RBC(VL)). Analyses of tryptic fragments by mass spectrometry led to the identification of two high-molecular weight 9-O-acetylated sialoglycoproteins as human erythrocytic α- and β-spectrin. Total spectrin purified from erythrocytes of VL patients (spectrin(VL)) was reactive with Achatinin-H. Interestingly, along with two high molecular weight bands corresponding to α- and β-spectrin another low molecular weight 60 kDa band was observed. Total spectrin was also purified from normal human erythrocytes (spectrin(N)) and insignificant binding with Achatinin-H was demonstrated. Additionally, this 60 kDa fragment was totally absent in spectrin(N). Although the presence of both N- and O-glycosylations was found both in spectrin(N) and spectrin(VL), enhanced sialylation was predominantly induced in spectrin(VL). Sialic acids accounted for approximately 1.25 kDa mass of the 60 kDa polypeptide. The demonstration of a few identified sialylated tryptic fragments of α- and β-spectrin(VL) confirmed the presence of terminal sialic acids. Molecular modelling studies of spectrin suggest that a sugar moiety can fit into the potential glycosylation sites. Interestingly, highly sialylated spectrin(VL) showed decreased binding with spectrin-depleted inside-out membrane vesicles of normal erythrocytes compared to spectrin(N) suggesting functional abnormality. Taken together this is the first report of glycosylated eythrocytic spectrin in normal erythrocytes and its enhanced sialylation in RBC(VL). The enhanced sialylation of this cytoskeleton protein is possibly related to the fragmentation of spectrin(VL) as evidenced by the presence of an additional 60 kDa fragment, absent in spectrin(N) which possibly affects the biology of RBC(VL) linked to both severe distortion of erythrocyte development and impairment of erythrocyte membrane integrity and may provide an explanation for their sensitivity to hemolysis and anemia in VL patients.
Conflict of interest statement
Figures





References
-
- Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–1392. - PubMed
-
- Bossi D, Russo M. Hemolytic anemias due to disorders of red cell membrane skeleton. Mol Aspects Med. 1996;17:171–188. - PubMed
-
- Resmi H, Pekçetin Ç, Güner G. Erythrocyte membrane and cytoskeletal protein glycation and oxidation in short-term diabetic rabbits. Clin Exp Med. 2001;1:187–193. - PubMed
-
- Starodubtseva MN, Kuznetsova TG, Yegorenkov NI, Cherenkevich SN. Structural and mechanical characteristics of erythrocyte membranes in patients with type 2 diabetes mellitus. Bull Exp Biol Med. 2008;145:99–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

