Monkeypox disease transmission in an experimental setting: prairie dog animal model
- PMID: 22164263
- PMCID: PMC3229555
- DOI: 10.1371/journal.pone.0028295
Monkeypox disease transmission in an experimental setting: prairie dog animal model
Abstract
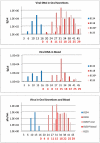
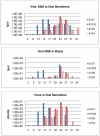
Monkeypox virus (MPXV) is considered the most significant human public health threat in the genus Orthopoxvirus since the eradication of variola virus (the causative agent of smallpox). MPXV is a zoonotic agent endemic to forested areas of Central and Western Africa. In 2003, MPXV caused an outbreak in the United States due to the importation of infected African rodents, and subsequent sequential infection of North American prairie dogs (Cynomys ludovicianus) and humans. In previous studies, the prairie dog MPXV model has successfully shown to be very useful for understanding MPXV since the model emulates key characteristics of human monkeypox disease. In humans, percutaneous exposure to animals has been documented but the primary method of human-to-human MPXV transmission is postulated to be by respiratory route. Only a few animal model studies of MPXV transmission have been reported. Herein, we show that MPXV infected prairie dogs are able to transmit the virus to naive animals through multiple transmission routes. All secondarily exposed animals were infected with MPXV during the course of the study. Notably, animals secondarily exposed appeared to manifest more severe disease; however, the disease course was very similar to those of experimentally challenged animals including inappetence leading to weight loss, development of lesions, production of orthopoxvirus antibodies and shedding of similar levels or in some instances higher levels of MPXV from the oral cavity. Disease was transmitted via exposure to contaminated bedding, co-housing, or respiratory secretions/nasal mucous (we could not definitively say that transmission occurred via respiratory route exclusively). Future use of the model will allow us to evaluate infection control measures, vaccines and antiviral strategies to decrease disease transmission.
Conflict of interest statement
Figures
References
-
- Breman JG, Nakano JH, Coffi E, Godfrey H, Gautun JC. Human poxvirus disease after smallpox eradication. Am J Trop Med Hyg. 1977;26:273–281. - PubMed
-
- Khodakevich L, Szczeniowski M, Manbu mD, Jezek Z, Marennikova S, et al. The role of squirrels in sustaining monkeypox virus transmission. Trop Geogr Med. 1987;39:115–122. - PubMed
-
- Centers for Disease Control and Prevention. Update: multistate outbreak of monkeypox–Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:561–564. - PubMed
-
- Hutson CL, Lee KN, Abel J, Carroll DS, Montgomery JM, et al. Monkeypox zoonotic associations: insights from laboratory evaluation of animals associated with the multi-state US outbreak. Am J Trop Med Hyg. 2007;76:757–768. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

