Publication delay of randomized trials on 2009 influenza A (H1N1) vaccination
- PMID: 22164274
- PMCID: PMC3229554
- DOI: 10.1371/journal.pone.0028346
Publication delay of randomized trials on 2009 influenza A (H1N1) vaccination
Abstract
Background: Randomized evidence for vaccine immunogenicity and safety is urgently needed in the setting of pandemics with new emerging infectious agents. We carried out an observational survey to evaluate how many randomized controlled trials testing 2009 H1N1 vaccines were published among those registered, and what was the time lag from their start to publication and from their completion to publication.
Methods: PubMed, EMBASE and 9 clinical trial registries were searched for eligible randomized controlled trials. The units of the analysis were single randomized trials on any individual receiving influenza vaccines in any setting.
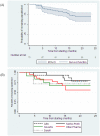
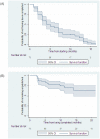
Results: 73 eligible trials were identified that had been registered in 2009-2010. By June 30, 2011 only 21 (29%) of these trials had been published, representing 38% of the randomized sample size (19905 of 52765). Trials starting later were published less rapidly (hazard ratio 0.42 per month; 95% Confidence Interval: 0.27 to 0.64; p<0.001). Similarly, trials completed later were published less rapidly (hazard ratio 0.43 per month; 95% CI: 0.27 to 0.67; p<0.001). Randomized controlled trials were completed promptly (median, 5 months from start to completion), but only a minority were subsequently published.
Conclusions: Most registered randomized trials on vaccines for the H1N1 pandemic are not published in the peer-reviewed literature.
Conflict of interest statement
Figures


References
-
- European Medicines Agency. Pandemic influenza A(H1N1)v vaccines authorised via the core dossier procedure. Explanatory note on scientific considerations regarding the licensing of pandemic A(H1N1)v vaccines. EMEA/608259/2009 rev. 2009. http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2009/11... (accessed 4 October 2011)
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

