In vitro model of vascularized bone: synergizing vascular development and osteogenesis
- PMID: 22164277
- PMCID: PMC3229596
- DOI: 10.1371/journal.pone.0028352
In vitro model of vascularized bone: synergizing vascular development and osteogenesis
Abstract
Tissue engineering provides unique opportunities for regenerating diseased or damaged tissues using cells obtained from tissue biopsies. Tissue engineered grafts can also be used as high fidelity models to probe cellular and molecular interactions underlying developmental processes. In this study, we co-cultured human umbilical vein endothelial cells (HUVECs) and human mesenchymal stem cells (MSCs) under various environmental conditions to elicit synergistic interactions leading to the colocalized development of capillary-like and bone-like tissues. Cells were encapsulated at the 1:1 ratio in fibrin gel to screen compositions of endothelial growth medium (EGM) and osteogenic medium (OM). It was determined that, to form both tissues, co-cultures should first be supplied with EGM followed by a 1:1 cocktail of the two media types containing bone morphogenetic protein-2. Subsequent studies of HUVECs and MSCs cultured in decellularized, trabecular bone scaffolds for 6 weeks assessed the effects on tissue construct of both temporal variations in growth-factor availability and addition of fresh cells. The resulting grafts were implanted subcutaneously into nude mice to determine the phenotype stability and functionality of engineered vessels. Two important findings resulted from these studies: (i) vascular development needs to be induced prior to osteogenesis, and (ii) the addition of additional hMSCs at the osteogenic induction stage improves both tissue outcomes, as shown by increased bone volume fraction, osteoid deposition, close proximity of bone proteins to vascular networks, and anastomosis of vascular networks with the host vasculature. Interestingly, these observations compare well with what has been described for native development. We propose that our cultivation system can mimic various aspects of endothelial cell-osteogenic precursor interactions in vivo, and could find utility as a model for studies of heterotypic cellular interactions that couple blood vessel formation with osteogenesis.
Conflict of interest statement
Figures



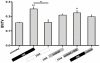

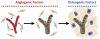
References
-
- Hofmann A, Ritz U, Verrier S, Eglin D, Alini M, et al. The effect of human osteoblasts on proliferation and neo-vessel formation of human umbilical vein endothelial cells in a long-term 3D co-culture on polyurethane scaffolds. Biomaterials. 2008;29:4217–4226. - PubMed
-
- Choong CSN, Hutmacher DW, Triffitt JT. Co-culture of bone marrow fibroblasts and endothelial cells on modified polycaprolactone substrates for enhanced potentials in bone tissue engineering. Tissue Engineering. 2006;12:2521–2531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

