Assessment of a novel VEGF targeted agent using patient-derived tumor tissue xenograft models of colon carcinoma with lymphatic and hepatic metastases
- PMID: 22164281
- PMCID: PMC3229582
- DOI: 10.1371/journal.pone.0028384
Assessment of a novel VEGF targeted agent using patient-derived tumor tissue xenograft models of colon carcinoma with lymphatic and hepatic metastases
Abstract
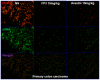
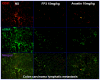
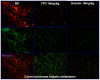
The lack of appropriate tumor models of primary tumors and corresponding metastases that can reliably predict for response to anticancer agents remains a major deficiency in the clinical practice of cancer therapy. It was the aim of our study to establish patient-derived tumor tissue (PDTT) xenograft models of colon carcinoma with lymphatic and hepatic metastases useful for testing of novel molecularly targeted agents. PDTT of primary colon carcinoma, lymphatic and hepatic metastases were used to create xenograft models. Hematoxylin and eosin staining, immunohistochemical staining, genome-wide gene expression analysis, pyrosequencing, qRT-PCR, and western blotting were used to determine the biological stability of the xenografts during serial transplantation compared with the original tumor tissues. Early passages of the PDTT xenograft models of primary colon carcinoma, lymphatic and hepatic metastases revealed a high degree of similarity with the original clinical tumor samples with regard to histology, immunohistochemistry, genes expression, and mutation status as well as mRNA expression. After we have ascertained that these xenografts models retained similar histopathological features and molecular signatures as the original tumors, drug sensitivities of the xenografts to a novel VEGF targeted agent, FP3 was evaluated. In this study, PDTT xenograft models of colon carcinoma with lymphatic and hepatic metastasis have been successfully established. They provide appropriate models for testing of novel molecularly targeted agents.
Conflict of interest statement
Figures






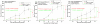




Similar articles
-
Antitumor effects of FP3 in combination with capecitabine on PDTT xenograft models of primary colon carcinoma and related lymphatic and hepatic metastases.Cancer Biol Ther. 2012 Jul;13(9):737-44. doi: 10.4161/cbt.20556. Epub 2012 May 23. Cancer Biol Ther. 2012. PMID: 22617773
-
Antitumor effect of FP3 in combination with cetuximab on patient-derived tumor tissue xenograft models of primary colon carcinoma and related lymphatic and hepatic metastases.Int J Mol Med. 2012 Jul;30(1):126-32. doi: 10.3892/ijmm.2012.968. Epub 2012 Apr 10. Int J Mol Med. 2012. PMID: 22505231
-
Differential response to EGFR- and VEGF-targeted therapies in patient-derived tumor tissue xenograft models of colon carcinoma and related metastases.Int J Oncol. 2012 Aug;41(2):583-8. doi: 10.3892/ijo.2012.1469. Epub 2012 May 10. Int J Oncol. 2012. PMID: 22581265
-
Heterogeneity-related anticancer therapy response differences in metastatic colon carcinoma: new hints to tumor-site-based personalized cancer therapy.Hepatogastroenterology. 2013 Nov-Dec;60(128):1927-34. Hepatogastroenterology. 2013. PMID: 24719929
-
Establishment of a PDTT xenograft model of gastric carcinoma and its application in personalized therapeutic regimen selection.Hepatogastroenterology. 2011 Sep-Oct;58(110-111):1814-22. doi: 10.5754/hge11136. Epub 2011 Jul 15. Hepatogastroenterology. 2011. PMID: 21940303
Cited by
-
The roles of patient-derived xenograft models and artificial intelligence toward precision medicine.MedComm (2020). 2024 Sep 25;5(10):e745. doi: 10.1002/mco2.745. eCollection 2024 Oct. MedComm (2020). 2024. PMID: 39329017 Free PMC article. Review.
-
Colon cancers carrying BRAF V600E and β-catenin T41A activating mutations are resistant to numerous common anticancer drugs.Oncol Lett. 2018 Apr;15(4):4471-4476. doi: 10.3892/ol.2018.7856. Epub 2018 Jan 25. Oncol Lett. 2018. PMID: 29541216 Free PMC article.
-
Antitumor effect of FP3 in a breast cancer xenograft model.Exp Ther Med. 2013 Jan;5(1):85-88. doi: 10.3892/etm.2012.773. Epub 2012 Oct 26. Exp Ther Med. 2013. PMID: 23251246 Free PMC article.
-
Patient-derived xenograft models for gastrointestinal tumors: A single-center retrospective study.Front Oncol. 2022 Nov 18;12:985154. doi: 10.3389/fonc.2022.985154. eCollection 2022. Front Oncol. 2022. PMID: 36465411 Free PMC article.
-
Luteolin exerts a marked antitumor effect in cMet-overexpressing patient-derived tumor xenograft models of gastric cancer.J Transl Med. 2015 Feb 1;13:42. doi: 10.1186/s12967-015-0398-z. J Transl Med. 2015. PMID: 25638174 Free PMC article.
References
-
- Morton CL, Houghton PJ. Establishment of human tumor xenografts in immunodeficient mice. Nat Protoc. 2007;2:247–250. - PubMed
-
- Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res. 2003;9:4227–4239. - PubMed
-
- Sausville EA, Burger AM. Contributions of human tumor xenografts to anticancer drug development. Cancer Res. 2006;66:3351–3354. - PubMed
-
- Jin K, He K, Teng F, Han N, Li G, et al. Heterogeneity in primary tumors and corresponding metastases: could it provide us with any hints to personalize cancer therapy? Pers Med. 2011;8:175–182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

