Using the indirect cohort design to estimate the effectiveness of the seven valent pneumococcal conjugate vaccine in England and Wales
- PMID: 22164292
- PMCID: PMC3229580
- DOI: 10.1371/journal.pone.0028435
Using the indirect cohort design to estimate the effectiveness of the seven valent pneumococcal conjugate vaccine in England and Wales
Abstract
Background: The 7-valent pneumococcal conjugate vaccine (PCV-7) was introduced in the United Kingdom in 2006 with a 2, 3 and 13 month schedule, and has led to large decreases in invasive pneumococcal disease (IPD) caused by the vaccine serotypes in both vaccinated and unvaccinated cohorts. We estimated the effectiveness of PCV-7 against IPD.
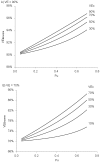
Methods and findings: We used enhanced surveillance data, collated at the Health Protection Agency, on vaccine type (n = 153) and non vaccine type (n = 919) IPD cases eligible for PCV-7. The indirect cohort method, a case-control type design which uses non vaccine type cases as controls, was used to estimate effectiveness of various numbers of doses as well as for each vaccine serotype. Possible bias with this design, caused by differential serotype replacement in vaccinated and unvaccinated individuals, was estimated after deriving formulae to quantify the bias. The results showed good effectiveness, increasing from 56% (95% confidence interval (CI): -7-82) for a single dose given under one year of age to 93% (95% CI: 70-98) for two doses under one year of age plus a booster dose in the second year of life. Serotype specific estimates indicated higher effectiveness against serotypes 4, 14 and 18C and lower effectiveness against 6B. Under the assumption of complete serotype replacement by non vaccine serotypes in carriage, we estimated that effectiveness estimates may be overestimated by about 2 to 5%.
Conclusions: This study shows high effectiveness of PCV-7 under the reduced schedule used in the UK. This finding agrees with the large reductions seen in vaccine type IPD in recent years in England and Wales. The formulae derived to assess the bias of the indirect cohort method for PCV-7 can also be used when using the design for other vaccines that affect carriage such as the recently introduced 13 valent pneumococcal conjugate vaccine.
Conflict of interest statement
Figures

References
-
- Goldblatt D, Southern J, Ashton L, Richmond P, Burbidge P, et al. Immunogenicity and Boosting after a Reduced Number of Doses of a Pneumococal Conjugate Vaccine in Infants and Toddlers. Pediatr Infect Dis J. 2006;25:312–319. - PubMed
-
- Miller E, Andrews NJ, Waight PA, Slack MPE, George RC. Herd immunity and serotype replacement four years after pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11:760–8. - PubMed
-
- Salisbury DS. 2010. Changes to the childhood pneumococcal conjugate vaccine. Available: http://www.dh.gov.uk/en/Publicationsandstatistics/Lettersandcirculars/De.... Accessed 28 July 2011.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

