Persistent expression of hepatitis C virus non-structural proteins leads to increased autophagy and mitochondrial injury in human hepatoma cells
- PMID: 22164304
- PMCID: PMC3229600
- DOI: 10.1371/journal.pone.0028551
Persistent expression of hepatitis C virus non-structural proteins leads to increased autophagy and mitochondrial injury in human hepatoma cells
Abstract
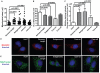
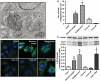
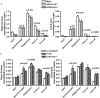
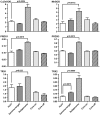
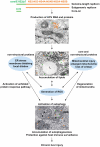
HCV infection is a major cause of chronic liver disease and liver cancer in the United States. To address the pathogenesis caused by HCV infection, recent studies have focused on the direct cytopathic effects of individual HCV proteins, with the objective of identifying their specific roles in the overall pathogenesis. However, this approach precludes examination of the possible interactions between different HCV proteins and organelles. To obtain a better understanding of the various cytopathic effects of and cellular responses to HCV proteins, we used human hepatoma cells constitutively replicating HCV RNA encoding either the full-length polyprotein or the non-structural proteins, or cells constitutively expressing the structural protein core, to model the state of persistent HCV infection and examined the combination of various HCV proteins in cellular pathogenesis. Increased reactive oxygen species (ROS) generation in the mitochondria, mitochondrial injury and degeneration, and increased lipid accumulation were common among all HCV protein-expressing cells regardless of whether they expressed the structural or non-structural proteins. Expression of the non-structural proteins also led to increased oxidative stress in the cytosol, membrane blebbing in the endoplasmic reticulum, and accumulation of autophagocytic vacuoles. Alterations of cellular redox state, on the other hand, significantly changed the level of autophagy, suggesting a direct link between oxidative stress and HCV-mediated activation of autophagy. With the wide-spread cytopathic effects, cells with the full-length HCV polyprotein showed a modest antioxidant response and exhibited a significant increase in population doubling time and a concomitant decrease in cyclin D1. In contrast, cells expressing the non-structural proteins were able to launch a vigorous antioxidant response with up-regulation of antioxidant enzymes. The population doubling time and cyclin D1 level were also comparable to that of control cells. Finally, the cytopathic effects of core protein appeared to focus on the mitochondria without remarkable disturbances in the cytosol.
Conflict of interest statement
Figures

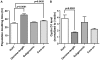
Similar articles
-
Oxidative Stress Attenuates Lipid Synthesis and Increases Mitochondrial Fatty Acid Oxidation in Hepatoma Cells Infected with Hepatitis C Virus.J Biol Chem. 2016 Jan 22;291(4):1974-1990. doi: 10.1074/jbc.M115.674861. Epub 2015 Dec 1. J Biol Chem. 2016. PMID: 26627833 Free PMC article.
-
Hepatocarcinogenesis in hepatitis C: HCV shrewdly exacerbates oxidative stress by modulating both production and scavenging of reactive oxygen species.Oncology. 2011;81 Suppl 1:11-7. doi: 10.1159/000333253. Epub 2011 Dec 22. Oncology. 2011. PMID: 22212930
-
Hepatitis C virus triggers mitochondrial permeability transition with production of reactive oxygen species, leading to DNA damage and STAT3 activation.J Virol. 2006 Jul;80(14):7199-207. doi: 10.1128/JVI.00321-06. J Virol. 2006. PMID: 16809325 Free PMC article.
-
Mitochondrial damage and iron metabolic dysregulation in hepatitis C virus infection.Free Radic Biol Med. 2019 Mar;133:193-199. doi: 10.1016/j.freeradbiomed.2018.09.044. Epub 2018 Sep 27. Free Radic Biol Med. 2019. PMID: 30268888 Review.
-
Hepatitis C virus contributes to hepatocarcinogenesis by modulating metabolic and intracellular signaling pathways.J Gastroenterol Hepatol. 2007 Jun;22 Suppl 1:S108-11. doi: 10.1111/j.1440-1746.2006.04669.x. J Gastroenterol Hepatol. 2007. PMID: 17567457 Review.
Cited by
-
HCV-Mediated Apoptosis of Hepatocytes in Culture and Viral Pathogenesis.PLoS One. 2016 Jun 9;11(6):e0155708. doi: 10.1371/journal.pone.0155708. eCollection 2016. PLoS One. 2016. PMID: 27280444 Free PMC article.
-
Hepatitis C virus induces the mitochondrial translocation of Parkin and subsequent mitophagy.PLoS Pathog. 2013 Mar;9(3):e1003285. doi: 10.1371/journal.ppat.1003285. Epub 2013 Mar 28. PLoS Pathog. 2013. PMID: 23555273 Free PMC article.
-
Role of mitochondria in parvovirus pathology.PLoS One. 2014 Jan 21;9(1):e86124. doi: 10.1371/journal.pone.0086124. eCollection 2014. PLoS One. 2014. PMID: 24465910 Free PMC article.
-
HCV and Oxidative Stress: Implications for HCV Life Cycle and HCV-Associated Pathogenesis.Oxid Med Cell Longev. 2016;2016:9012580. doi: 10.1155/2016/9012580. Epub 2016 Feb 3. Oxid Med Cell Longev. 2016. PMID: 26955431 Free PMC article. Review.
-
Functions of autophagy in normal and diseased liver.Autophagy. 2013 Aug;9(8):1131-58. doi: 10.4161/auto.25063. Epub 2013 May 22. Autophagy. 2013. PMID: 23774882 Free PMC article. Review.
References
-
- Robertson B, Myers G, Howard C, Brettin T, Bukh J, et al. Classification, nomenclature, and database development for hepatitis C virus (HCV) and related viruses: proposals for standardization. International Committee on Virus Taxonomy. Arch Virol. 1998;143:2493–2503. - PubMed
-
- Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5:453–463. - PubMed
-
- Branch AD, Stump DD, Gutierrez JA, Eng F, Walewski JL. The hepatitis C virus alternate reading frame (ARF) and its family of novel products: the alternate reading frame protein/F-protein, the double-frameshift protein, and others. Semin Liver Dis. 2005;25:105–117. - PubMed
-
- Georgel P, Schuster C, Zeisel MB, Stoll-Keller F, Berg T, et al. Virus-host interactions in hepatitis C virus infection: implications for molecular pathogenesis and antiviral strategies. Trends Mol Med. 2010;16:277–286. - PubMed
-
- Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946–952. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

