Dose-Dependent Change in Elimination Kinetics of Ethanol due to Shift of Dominant Metabolizing Enzyme from ADH 1 (Class I) to ADH 3 (Class III) in Mouse
- PMID: 22164338
- PMCID: PMC3227458
- DOI: 10.1155/2012/408190
Dose-Dependent Change in Elimination Kinetics of Ethanol due to Shift of Dominant Metabolizing Enzyme from ADH 1 (Class I) to ADH 3 (Class III) in Mouse
Abstract
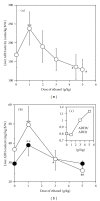
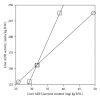
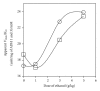
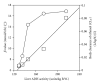
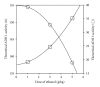
ADH 1 and ADH 3 are major two ADH isozymes in the liver, which participate in systemic alcohol metabolism, mainly distributing in parenchymal and in sinusoidal endothelial cells of the liver, respectively. We investigated how these two ADHs contribute to the elimination kinetics of blood ethanol by administering ethanol to mice at various doses, and by measuring liver ADH activity and liver contents of both ADHs. The normalized AUC (AUC/dose) showed a concave increase with an increase in ethanol dose, inversely correlating with β. CL(T) (dose/AUC) linearly correlated with liver ADH activity and also with both the ADH-1 and -3 contents (mg/kg B.W.). When ADH-1 activity was calculated by multiplying ADH-1 content by its V(max)/mg (4.0) and normalized by the ratio of liver ADH activity of each ethanol dose to that of the control, the theoretical ADH-1 activity decreased dose-dependently, correlating with β. On the other hand, the theoretical ADH-3 activity, which was calculated by subtracting ADH-1 activity from liver ADH activity and normalized, increased dose-dependently, correlating with the normalized AUC. These results suggested that the elimination kinetics of blood ethanol in mice was dose-dependently changed, accompanied by a shift of the dominant metabolizing enzyme from ADH 1 to ADH 3.
Figures




References
-
- Lumeng L, Bosron WF, Li TK. Quantitative correlation of ethanol elimination rates in vivo with liver alcohol dehydrogenase activities in fed, fasted and food-restricted rats. Biochemical Pharmacology. 1979;28(9):1547–1551. - PubMed
-
- Crow KE, Hardman MJ. Regulation of rates of ethanol metabolism. In: Crow KE, Batt RD, editors. Regulation of Rates of Ethanol Metabolism. in Human Metabolism of Alcohol, Regulation, Enzymology, and Metabolites of Ethanol. Vol. 11. Boca Raton, Fla, USA: CRC Press; 1989. pp. 3–16.
-
- Haseba T, Kurosu M, Ohno Y. Dose- and time-dependent changes of mouse liver alcohol dehydrogenase (ADH) activity involving both Class I and Class III ADHs after administration of ethanol. Legal Medicine. 2003:202–211. - PubMed
-
- Plapp BV, Leidal KG, Smith RK, Murch BP. Kinetics of inhibition of ethanol metabolism in rats and the rate-limiting role of alcohol dehydrogenase. Archives of Biochemistry and Biophysics. 1984;230(1):30–38. - PubMed
LinkOut - more resources
Full Text Sources

