Phase II trial of the regulatory T cell-depleting agent, denileukin diftitox, in patients with unresectable stage IV melanoma
- PMID: 22165955
- PMCID: PMC3293785
- DOI: 10.1186/1471-2407-11-515
Phase II trial of the regulatory T cell-depleting agent, denileukin diftitox, in patients with unresectable stage IV melanoma
Abstract
Background: We previously found that administration of an interleukin 2/diphtheria toxin conjugate (DAB/IL2; Denileukin Diftitox; ONTAK) to stage IV melanoma patients depleted CD4(+)CD25(HI)Foxp3(+) regulatory T cells and expanded melanoma-specific CD8(+) T cells. The goal of this study was to assess the clinical efficacy of DAB/IL2 in an expanded cohort of stage IV melanoma patients.
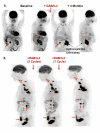
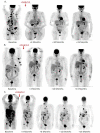
Methods: In a single-center, phase II trial, DAB/IL2 (12 μg/kg; 4 daily doses; 21 day cycles) was administered to 60 unresectable stage IV melanoma patients and response rates were assessed using a combination of 2-[(18)F]-fluoro-2-deoxy-glucose (FDG)-positron emission tomography (PET) and computed tomography (CT) imaging.
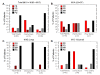
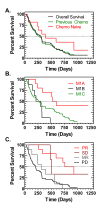
Results: After DAB/IL2 administration, 16.7% of the 60 patients had partial responses, 5% stable disease and 15% mixed responses. Importantly, 45.5% of the chemo/immuno-naïve sub-population (11/60 patients) experienced partial responses. One year survival was markedly higher in partial responders (80 ± 11.9%) relative to patients with progressive disease (23.7 ± 6.5%; p value < 0.001) and 40 ± 6.2% of the total DAB/IL2-treated population were alive at 1 year.
Conclusions: These data support the development of multi-center, randomized trials of DAB/IL2 as a monotherapy and in combination with other immunotherapeutic agents for the treatment of stage IV melanoma.
Trial registration: NCT00299689.
Figures
References
-
- Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ, Moon J, Sondak VK, Atkins MB, Eisenhauer EA. et al.Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26(4):527–534. - PubMed
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. - PubMed
-
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M. et al.High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105–2116. - PubMed
-
- Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14(1):7–17. - PubMed
-
- Kirkwood JM, Ibrahim JG, Sondak VK, Richards J, Flaherty LE, Ernstoff MS, Smith TJ, Rao U, Steele M, Blum RH. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000;18(12):2444–2458. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

