Evaluation of the cell viability of human Wharton's jelly stem cells for use in cell therapy
- PMID: 22166141
- PMCID: PMC3358099
- DOI: 10.1089/ten.TEC.2011.0508
Evaluation of the cell viability of human Wharton's jelly stem cells for use in cell therapy
Abstract
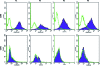
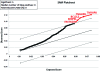
Human umbilical cord Wharton's jelly stem cells (HWJSCs) are gaining attention as a possible clinical source of mesenchymal stem cells for cell therapy and tissue engineering due to their high accessibility, expansion potential, and plasticity. We employed a combination of highly sensitive techniques to determine the average cell viability levels and proliferation capabilities of 10 consecutive cell passages of cultured HWJSCs and then used RNA microarrays to identify genes associated with changes in cell viability levels. We found an initial decrease in cell viability from the first to the third cell passage followed by an increase until the sixth passage and a final decrease from the sixth to tenth cell passages. The highest cell viability levels corresponded to the fifth and sixth passages. The intracellular ionic contents of potassium, sodium, and chlorine suggest that the lower cell viability levels at passages 2, 3, and 8-10 may be associated with apoptotic cell death. In fact, gene expression analysis revealed that the average cell viability was significantly associated with genes with a function in apoptotic cell death, especially pro-apoptotic FASTKD2, BNIP3L genes and anti-apoptotic TNFAIP8 and BCL2L2 genes. This correlation with both pro-apoptotic and anti-apoptotic genes suggests that there may be a complex live-death equilibrium in cultured HWJSCs kept in culture for multiple cell passages. In this study, the highest cell viability levels corresponded to the fifth and sixth HWJSC passages, suggesting that these passages should be preferentially employed in cell therapy or tissue engineering protocols using this cell type.
Figures



References
-
- Alaminos M. Perez-Kohler B. Garzon I. Garcia-Honduvilla N. Romero B. Campos A. Bujan J. Transdifferentiation potentiality of human Wharton's jelly stem cells towards vascular endothelial cells. J Cell Physiol. 2010;223:640. - PubMed
-
- Wu K.H. Zhou B. Lu S.H. Feng B. Yang S.G. Du W.T. Gu D.S. Han Z.C. Liu Y.L. In vitro and in vivo differentiation of human umbilical cord derived stem cells into endothelial cells. J Cell Biochem. 2007;100:608. - PubMed
-
- Chang Y.S. Oh W. Choi S.J. Sung D.K. Kim S.Y. Choi E.Y. Kang S. Jin H.J. Yang Y.S. Park W.S. Human umbilical cord blood-derived mesenchymal stem cells attenuate hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2009;18:869. - PubMed
-
- Can A. Karahuseyinoglu S. Concise review: human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 2007;25:2886. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

