Weak coordination as a powerful means for developing broadly useful C-H functionalization reactions
- PMID: 22166158
- PMCID: PMC3334399
- DOI: 10.1021/ar200185g
Weak coordination as a powerful means for developing broadly useful C-H functionalization reactions
Abstract
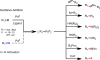
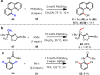
Reactions that convert carbon-hydrogen (C-H) bonds into carbon-carbon (C-C) or carbon-heteroatom (C-Y) bonds are attractive tools for organic chemists, potentially expediting the synthesis of target molecules through new disconnections in retrosynthetic analysis. Despite extensive inorganic and organometallic study of the insertion of homogeneous metal species into unactivated C-H bonds, practical applications of this technology in organic chemistry are still rare. Only in the past decade have metal-catalyzed C-H functionalization reactions become more widely utilized in organic synthesis. Research in the area of homogeneous transition metal-catalyzed C-H functionalization can be broadly grouped into two subfields. They reflect different approaches and goals and thus have different challenges and opportunities. One approach involves reactions of completely unfunctionalized aromatic and aliphatic hydrocarbons, which we refer to as "first functionalization". Here the substrates are nonpolar and hydrophobic and thus interact very weakly with polar metal species. To overcome this weak affinity and drive metal-mediated C-H cleavage, chemists often use hydrocarbon substrates in large excess (for example, as solvent). Because highly reactive metal species are needed in first functionalization, controlling the chemoselectivity to avoid overfunctionalization is often difficult. Additionally, because both substrates and products are comparatively low-value chemicals, developing cost-effective catalysts with exceptionally high turnover numbers that are competitive with alternatives (including heterogeneous catalysts) is challenging. Although an exciting field, first functionalization is beyond the scope of this Account. The second subfield of C-H functionalization involves substrates containing one or more pre-existing functional groups, termed "further functionalization". One advantage of this approach is that the existing functional group (or groups) can be used to chelate the metal catalyst and position it for selective C-H cleavage. Precoordination can overcome the paraffin nature of C-H bonds by increasing the effective concentration of the substrate so that it need not be used as solvent. From a synthetic perspective, it is desirable to use a functional group that is an intrinsic part of the substrate so that extra steps for installation and removal of an external directing group can be avoided. In this way, dramatic increases in molecular complexity can be accomplished in a single stroke through stereo- and site-selective introduction of a new functional group. Although reactivity is a major challenge (as with first functionalization), the philosophy in further functionalization differs; the major challenge is developing reactions that work with predictable selectivity in intricately functionalized contexts on commonly occurring structural motifs. In this Account, we focus on an emergent theme within the further functionalization literature: the use of commonly occurring functional groups to direct C-H cleavage through weak coordination. We discuss our motivation for studying Pd-catalyzed C-H functionalization assisted by weakly coordinating functional groups and chronicle our endeavors to bring reactions of this type to fruition. Through this approach, we have developed reactions with a diverse range of substrates and coupling partners, with the broad scope likely stemming from the high reactivity of the cyclopalladated intermediates, which are held together through weak interactions.
Figures
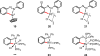
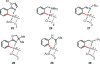
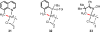
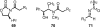
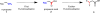
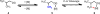
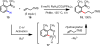

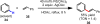

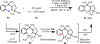


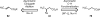
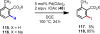
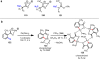
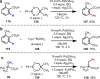

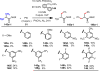
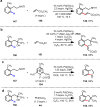
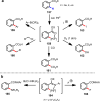
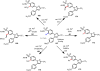


References
-
- Engle KM, Yu J-Q. Transition Metal–Catalyzed C–H Functionalization: Synthetically Enabling Reactions for Building Molecular Complexity. Weinheim, Germany; Wiley: 2011.
-
- Murai S, Kakiuchi F, Sekine S, Tanaka Y, Kamatani A, Sonoda M, Chatani N. Efficient catalytic addition of aromatic carbon-hydrogen bonds to olefins. Nature. 1993;366:529–531.
-
- Trost BM, Imi K, Davies IW. Elaboration of Conjugated Alkenes Initiated by Insertion into a Vinylic C-H Bond. J. Am. Chem. Soc. 1995;117:5371–5372.
-
- Lenges CP, Brookhart M. Addition of Olefins to Aromatic Ketones Catalyzed by Rh(I) Olefin Complexes. J. Am. Chem. Soc. 1999;121:6616–6623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

