Enzymatic synthesis of substituted epicatechins for bioactivity studies in neurological disorders
- PMID: 22166210
- PMCID: PMC5470586
- DOI: 10.1016/j.bbrc.2011.11.139
Enzymatic synthesis of substituted epicatechins for bioactivity studies in neurological disorders
Erratum in
- Biochem Biophys Res Commun. 2015 May 1;460(2):489
Abstract
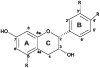
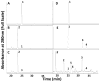
Glucuronidated and/or methylated metabolites of the proanthocyanidin (PA) monomer (-)-epicatechin are detected in both blood and brain following feeding of rodents with a monomeric grape seed PA extract shown to reduce symptoms in a mouse model of Alzheimer's disease. To generate metabolites for future mechanistic studies, we investigated the ability of recombinant human glucuronosyl transferases of the UGT1A and UGT2B families to glucuronidate epicatechin or 3'-O-methyl epicatechin in vitro. Of twelve enzymes tested, UGT1A9 was the most efficient, producing epicatechin 3'-O-glucuronide as the major product. Incubation of UGT1A9 with 3'-O-methyl-epicatechin resulted in two major products, one of which was identified as 3'-O-methyl-epicatechin 5-O-glucuronide, a major metabolite found in blood plasma and brain tissue of the rodents following feeding with a grape seed extract. We also investigated in vitro methylation of epicatechin and epicatechin glucuronides by human catechol O-methyltransferase. Enzymatic production of 3'-O-methyl-epicatechin 5-O-glucuronide was optimized to 50% overall yield. These studies form a basis for generation of mg quantities of pure epicatechin (methyl) glucuronides of biological significance, and provide clarification of structure of previously identified epicatechin metabolites.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



References
-
- Abd-el-Mohsen M, Kuhnle G, Rechner A, Schroeter H, Rose S, Jenner P, Rice-Evans C. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radical Biology and Medicine. 2002;33:1693–1702. - PubMed
-
- Baba S, Osakabe N, Natsume M, Muto Y, Takizawa T, Terao J. In vivo comparison of the bioavailability of (1)-catechin, (2)-epicatechin and their mixture in orally administered rats. Journal of Nutrition. 2001;131:2885–2891. - PubMed
-
- Baba S, Osakabe N, Natsume M, Yasuda A, Takizawa T. Cocoa powder enhances the level of antioxidative activity in rat plasma. British Journal of Nutrition. 2000;84:673–680. - PubMed
-
- Feng W. Metabolism of green tea catechins: an overview. Current Drug Metabolism. 2006;7:755–809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

