Autophagy and cancer
- PMID: 22166310
- PMCID: PMC3249624
- DOI: 10.1101/cshperspect.a008821
Autophagy and cancer
Abstract
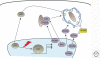
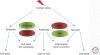
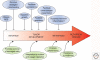
(Macro)autophagy is a cellular membrane trafficking process that serves to deliver cytoplasmic constituents to lysosomes for degradation. At basal levels, it is critical for maintaining cytoplasmic as well as genomic integrity and is therefore key to maintaining cellular homeostasis. Autophagy is also highly adaptable and can be modified to digest specific cargoes to bring about selective effects in response to numerous forms of intracellular and extracellular stress. It is not a surprise, therefore, that autophagy has a fundamental role in cancer and that perturbations in autophagy can contribute to malignant disease. We review here the roles of autophagy in various aspects of tumor suppression including the response of cells to nutrient and hypoxic stress, the control of programmed cell death, and the connection to tumor-associated immune responses.
Figures

References
-
- Adams PD 2009. Healing and hurting: Molecular mechanisms, functions, and pathologies of cellular senescence. Mol Cell 36: 2–14 - PubMed
-
- Aita VM, Liang XH, Murty VV, Pincus DL, Yu W, Cayanis E, Kalachikov S, Gilliam TC, Levine B 1999. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics 59: 59–65 - PubMed
-
- Aki T, Yamaguchi K, Fujimiya T, Mizukami Y 2003. Phosphoinositide 3-kinase accelerates autophagic cell death during glucose deprivation in the rat cardiomyocyte-derived cell line H9c2. Oncogene 22: 8529–8535 - PubMed
-
- Amaravadi RK, Thompson CB 2007. The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin Cancer Res 13: 7271–7279 - PubMed
-
- Balkwill F, Charles KA, Mantovani A 2005. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7: 211–217 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
