Balapiravir plus peginterferon alfa-2a (40KD)/ribavirin in a randomized trial of hepatitis C genotype 1 patients
- PMID: 22166557
- PMCID: PMC3739984
Balapiravir plus peginterferon alfa-2a (40KD)/ribavirin in a randomized trial of hepatitis C genotype 1 patients
Abstract
Introduction: Balapiravir (R1626, RG1626) is the prodrug of a nucleoside analogue inhibitor of the hepatitis C virus (HCV) RNA-dependent RNA polymerase (R1479, RG1479). This phase 2, double-blind international trial evaluated the optimal treatment regimen of balapiravir plus peginterferon alfa-2a (40KD)/ribavirin.
Material and methods: Treatment-naive genotype 1 patients (N = 516) were randomized to one of seven treatment groups in which they received balapiravir 500, 1,000, or 1,500 mg twice daily, peginterferon alfa-2a (40KD) 180 or 90 µg/week and ribavirin 1,000/1,200 mg/day or peginterferon alfa-2a (40KD)/ribavirin. The planned treatment duration with balapiravir was reduced from 24 to 12 weeks due to safety concerns.
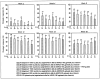
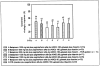
Results: The percentage of patients with undetectable HCV RNA was consistently higher in all balapiravir groups from week 2 to 12. However, high rates of dose modifications and discontinuations of one/all study drugs compromised the efficacy assessment and resulted in similar sustained virological response rates in the balapiravir groups (range 32-50%) and the peginterferon alfa-2a (40KD)/ribavirin group (43%). Balapiravir was discontinued for safety reasons in 28-36% of patients (most often for lymphopenia) and the percentage of patients with serious adverse events (especially hematological, infection, ocular events) was dose related. Serious hematological adverse events (particularly neutropenia, lymphopenia) were more common in balapiravir recipients. Two deaths in the balapiravir/peginterferon alfa-2a/ribavirin combination groups were considered possibly related to study medication.
Conclusion: Further development of balapiravir for the treatment of chronic hepatitis C has been halted because of the unacceptable benefit to risk ratio revealed in this study (www.ClinicalTrials.gov NCT 00517439).
Trial registration: ClinicalTrials.gov NCT00517439.
Figures



References
-
- Wasley A, Alter MJ. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin Liver Dis. 2000;20:1–16. - PubMed
-
- Kim WR. The burden of hepatitis C in the United States. Hepatology. 2002;36:S30–S34. - PubMed
-
- Klumpp K, Leveque V, Le Pogam S, Ma H, Jiang W-R, Kang H, Granycome C, et al. The novel nucleoside analog R1479 (4’-azidocytidine) is a potent inhibitor of NS5B-dependent RNA synthesis and hepatitis C virus replication in cell culture. J Biol Chem. 2006;281:3793–9. - PubMed
-
- Brandl M, Wu X, Holper M, Hong L, Jia Z, Birudaraj R, Reddy M, et al. Physicochemical properties of the nucleoside prodrug R1626 leading to high oral bioavailability. J Biol Chem. 2008;48:398–406. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

