Neuroprotective effects of recombinant T-cell receptor ligand in autoimmune optic neuritis in HLA-DR2 mice
- PMID: 22167100
- PMCID: PMC3292374
- DOI: 10.1167/iovs.11-8419
Neuroprotective effects of recombinant T-cell receptor ligand in autoimmune optic neuritis in HLA-DR2 mice
Abstract
Purpose: Optic neuritis (ON) is a condition involving primary inflammation, demyelination, and axonal injury in the optic nerve and leads to apoptotic retinal ganglion cell (RGC) death, which contributes to the persistence of visual loss. Currently, ON has no effective treatment. The goal was to determine the effectiveness of immunotherapy with recombinant T-cell receptor ligand (RTL) in preventing ON in humanized HLA-DR2 transgenic mice.
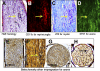
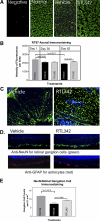
Methods: Experimental autoimmune encephalomyelitis (EAE) was induced with myelin oligodendrocyte glycoprotein in humanized HLA-DR2 (DRβ1*1501) transgenic mice. Five consecutive doses of RTL342M were administrated at the onset of ON. The development of autoimmune ON was assessed by histopathology at different time points. The levels of myelin loss, axonal loss, and RGC damage were examined by immunofluorescence.
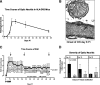
Results: HLA-DR2 mice developed chronic ON 2 days before EAE characterized by progressive neurodegeneration in both organs. RTL342M significantly suppressed inflammation in the optic nerve and spinal cord and provided protection for at least 30 days. Examination of myelin loss showed a marked suppression of demyelination and an increase in myelin recovery in the optic nerve. Moreover, RTL342M treatment revealed a neuroprotective effect on optic nerve axons and RGCs in retinas at postimmunization (PI) day 62.
Conclusions: RTL342M suppressed clinical and histologic signs of EAE/ON by preventing the recruitment of inflammatory cells into the optic nerve and showed neuroprotective effects against ON. However, to achieve full therapeutic benefit, more doses may be needed. These findings suggest a possible clinical application of this novel class of T-cell-tolerizing drugs for patients with optic neuritis.
Figures



References
-
- Margalit E, Sadda SR. Retinal and optic nerve diseases. Artif Organs. 2003;27:963–974 - PubMed
-
- Balcer LJ. Clinical practice: optic neuritis. N Engl J Med. 2006;354:1273–1280 - PubMed
-
- Wilejto M, Shroff M, Buncic JR, Kennedy J, Goia C, Banwell B. The clinical features, MRI findings, and outcome of optic neuritis in children. Neurology. 2006;67:258–262 - PubMed
-
- Shams PN, Plant GT. Optic neuritis: a review. Int MS J. 2009;16:82–89 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

