Endogenous vascular endothelial growth factor-A (VEGF-A) maintains endothelial cell homeostasis by regulating VEGF receptor-2 transcription
- PMID: 22167188
- PMCID: PMC3270960
- DOI: 10.1074/jbc.M111.293985
Endogenous vascular endothelial growth factor-A (VEGF-A) maintains endothelial cell homeostasis by regulating VEGF receptor-2 transcription
Abstract
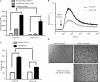
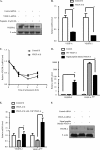
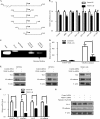
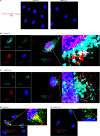
Vascular endothelial growth factor A (VEGF-A) is one of the most important factors controlling angiogenesis. Although the functions of exogenous VEGF-A have been widely studied, the roles of endogenous VEGF-A remain unclear. Here we focused on the mechanistic functions of endogenous VEGF-A in endothelial cells. We found that it is complexed with VEGF receptor 2 (VEGFR-2) and maintains a basal expression level for VEGFR-2 and its downstream signaling activation. Endogenous VEGF-A also controls expression of key endothelial specific genes including VEGFR-2, Tie-2, and vascular endothelial cadherin. Of importance, endogenous VEGF-A differs from exogenous VEGF-A by regulating VEGFR-2 transcription through mediation of FoxC2 binding to the FOX:ETS motif, and the complex formed by endogenous VEGF-A with VEGFR-2 is localized within the EEA1 (early endosome antigen 1) endosomal compartment. Taken together, our results emphasize the importance of endogenous VEGF-A in endothelial cells by regulating key vascular proteins and maintaining the endothelial homeostasis.
Figures

References
-
- Senger D. R., Galli S. J., Dvorak A. M., Perruzzi C. A., Harvey V. S., Dvorak H. F. (1983) Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219, 983–985 - PubMed
-
- Dvorak H. F., Nagy J. A., Feng D., Brown L. F., Dvorak A. M. (1999) Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr. Top. Microbiol. Immunol. 237, 97–132 - PubMed
-
- Nagy J. A., Vasile E., Feng D., Sundberg C., Brown L. F., Manseau E. J., Dvorak A. M., Dvorak H. F. (2002) VEGF-A induces angiogenesis, arteriogenesis, lymphangiogenesis, and vascular malformations. Cold Spring Harb. Symp. Quant. Biol. 67, 227–237 - PubMed
-
- Pettersson A., Nagy J. A., Brown L. F., Sundberg C., Morgan E., Jungles S., Carter R., Krieger J. E., Manseau E. J., Harvey V. S., Eckelhoefer I. A., Feng D., Dvorak A. M., Mulligan R. C., Dvorak H. F. (2000) Heterogeneity of the angiogenic response induced in different normal adult tissues by vascular permeability factor/vascular endothelial growth factor. Lab. Invest. 80, 99–115 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

