Ex vivo and in vivo effects of isofagomine on acid β-glucosidase variants and substrate levels in Gaucher disease
- PMID: 22167193
- PMCID: PMC3281716
- DOI: 10.1074/jbc.M111.280016
Ex vivo and in vivo effects of isofagomine on acid β-glucosidase variants and substrate levels in Gaucher disease
Abstract
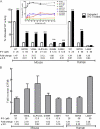
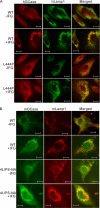
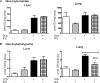
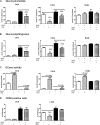
Isofagomine (IFG) is an acid β-glucosidase (GCase) active site inhibitor that acts as a pharmacological chaperone. The effect of IFG on GCase function was investigated in GCase mutant fibroblasts and mouse models. IFG inhibits GCase with K(i) ∼30 nM for wild-type and mutant enzymes (N370S and V394L). Fibroblasts treated with IFG at μM concentrations showed enhancement of WT and mutant GCase activities and protein levels. Administration of IFG (30 mg/kg/day) to the mice homozygous for GCase mutations (V394L, D409H, or D409V) led to increased GCase activity in visceral tissues and brain extracts. IFG effects on GCase stability and substrate levels were evaluated in a mouse model (hG/4L/PS-NA) that has doxycycline-controlled human WT GCase (hGCase) expression driven by a liver-specific promoter and is also homozygous for the IFG-responsive V394L GCase. Both human and mouse GCase activity and protein levels were increased in IFG-treated mice. The liver-secreted hGCase in serum was stabilized, and its effect on the lung and spleen involvement was enhanced by IFG treatment. In 8-week IFG-treated mice, the accumulated glucosylceramide and glucosylsphingosine were reduced by 75 and 33%, respectively. Decreases of storage cells were correlated with >50% reductions in substrate levels. These results indicate that IFG stabilizes GCase in tissues and serum and can reduce visceral substrates in vivo.
Figures



References
-
- Grabowski G. A., Petsko G., Kolodny E. H. (2010) in The Online Metabolic and Molecular Bases of Inherited Diseases (Valle D., Beaudet A. L., Vogelstein B., Kinzler K. W., Antonarakis S. E., Ballabio A., Scriver C. R., Sly W. S., Childs B., eds) 9th Ed., Chapter 146, McGraw-Hill, New York
-
- Horowitz M., Wilder S., Horowitz Z., Reiner O., Gelbart T., Beutler E. (1989) The human glucocerebrosidase gene and pseudogene: structure and evolution. Genomics 4, 87–96 - PubMed
-
- Kolodny E. H., Ullman M. D., Mankin H. J., Raghavan S. S., Topol J., Sullivan J. L. (1982) Phenotypic manifestations of Gaucher disease: clinical features in 48 biochemically verified type 1 patients and comment on type 2 patients. Prog. Clin. Biol. Res. 95, 33–65 - PubMed
-
- Volk B. W., Wallace B. J., Adachi M. (1967) Infantile Gaucher's disease: electron microscopic and histochemical studies of a cerebral biopsy. J. Neuropathol. Exp. Neurol. 26, 176–177 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

