NMDA-dependent mechanisms only affect the BOLD response in the rat dentate gyrus by modifying local signal processing
- PMID: 22167232
- PMCID: PMC3293123
- DOI: 10.1038/jcbfm.2011.182
NMDA-dependent mechanisms only affect the BOLD response in the rat dentate gyrus by modifying local signal processing
Abstract
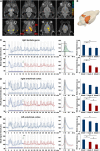
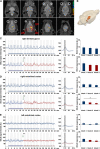
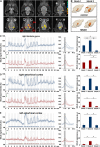
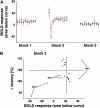
The role of N-methyl-D-aspartate (NMDA) receptor-mediated mechanisms in the formation of a blood oxygen level-dependent (BOLD) response was studied using electrical stimulation of the right perforant pathway. Stimulation of this fiber bundle triggered BOLD responses in the right hippocampal formation and in the left entorhinal cortex. The perforant pathway projects to and activates the dentate gyrus monosynaptically, activation in the contralateral entorhinal cortex is multisynaptic and requires forwarding and processing of signals. Application of the NMDA receptor antagonist MK801 during stimulation had no effect on BOLD responses in the right dentate gyrus, but reduced the BOLD responses in the left entorhinal cortex. In contrast, application of MK801 before the first stimulation train reduced the BOLD response in both regions. Electrophysiological recordings revealed that the initial stimulation trains changed the local processing of the incoming signals in the dentate gyrus. This altered electrophysiological response was not further changed by a subsequent application of MK801, which is in agreement with an unchanged BOLD response. When MK801 was present during the first stimulation train, a dissimilar electrophysiological response pattern was observed and corresponds to an altered BOLD response, indicating that NMDA-dependent mechanisms indirectly affect the BOLD response, mainly via modifying local signal processing and subsequent propagation.
Figures




References
-
- Angenstein F, Kammerer E, Niessen HG, Frey JU, Scheich H, Frey S. Frequency-dependent activation pattern in the rat hippocampus, a simultaneous electrophysiological and fMRI study. Neuroimage. 2007;38:150–163. - PubMed
-
- Angenstein F, Krautwald K, Scheich H. The current functional state of local neuronal circuits controls the magnitude of a BOLD response to incoming stimuli. Neuroimage. 2010;50:1364–1375. - PubMed
-
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

