CR8, a selective and potent CDK inhibitor, provides neuroprotection in experimental traumatic brain injury
- PMID: 22167461
- PMCID: PMC3324621
- DOI: 10.1007/s13311-011-0095-4
CR8, a selective and potent CDK inhibitor, provides neuroprotection in experimental traumatic brain injury
Abstract
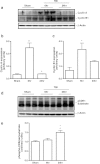
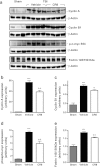
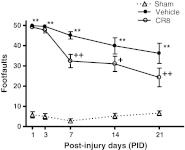
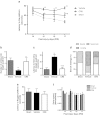
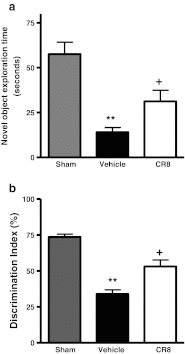
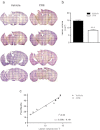
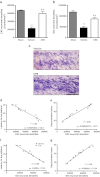
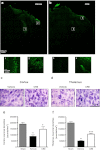
Traumatic brain injury (TBI) induces secondary injury mechanisms, including cell cycle activation (CCA), that leads to neuronal death and neurological dysfunction. We recently reported that delayed administration of roscovitine, a relatively selective cyclin-dependent kinase (CDK) inhibitor, inhibits CCA and attenuates neurodegeneration and functional deficits following controlled cortical impact (CCI) injury in mice. Here we evaluated the neuroprotective potential of CR8, a more potent second-generation roscovitine analog, using the mouse CCI model. Key CCA markers (cyclin A and B1) were significantly up-regulated in the injured cortex following TBI, and phosphorylation of CDK substrates was increased. Central administration of CR8 after TBI, at a dose 20 times less than previously required for roscovitine, attenuated CCA pathways and reduced post-traumatic apoptotic cell death at 24 h post-TBI. Central administration of CR8, at 3 h after TBI, significantly attenuated sensorimotor and cognitive deficits, decreased lesion volume, and improved neuronal survival in the cortex and dentate gyrus. Moreover, unlike roscovitine treatment in the same model, CR8 also attenuated post-traumatic neurodegeneration in the CA3 region of the hippocampus and thalamus at 21 days. Furthermore, delayed systemic administration of CR8, at a dose 10 times less than previously required for roscovitine, significantly improved cognitive performance after CCI. These findings further demonstrate the neuroprotective potential of cell cycle inhibitors following experimental TBI. Given the increased potency and efficacy of CR8 as compared to earlier purine analog types of CDK inhibitors, this drug should be considered as a candidate for future clinical trials of TBI.
Figures
Similar articles
-
CR8, a novel inhibitor of CDK, limits microglial activation, astrocytosis, neuronal loss, and neurologic dysfunction after experimental traumatic brain injury.J Cereb Blood Flow Metab. 2014 Mar;34(3):502-13. doi: 10.1038/jcbfm.2013.228. Epub 2014 Jan 8. J Cereb Blood Flow Metab. 2014. PMID: 24398934 Free PMC article.
-
Selective CDK inhibitor limits neuroinflammation and progressive neurodegeneration after brain trauma.J Cereb Blood Flow Metab. 2012 Jan;32(1):137-49. doi: 10.1038/jcbfm.2011.117. Epub 2011 Aug 10. J Cereb Blood Flow Metab. 2012. PMID: 21829212 Free PMC article.
-
Comparing effects of CDK inhibition and E2F1/2 ablation on neuronal cell death pathways in vitro and after traumatic brain injury.Cell Death Dis. 2018 Nov 6;9(11):1121. doi: 10.1038/s41419-018-1156-y. Cell Death Dis. 2018. PMID: 30401820 Free PMC article.
-
Roscovitine reduces neuronal loss, glial activation, and neurologic deficits after brain trauma.J Cereb Blood Flow Metab. 2008 Nov;28(11):1845-59. doi: 10.1038/jcbfm.2008.75. Epub 2008 Jul 9. J Cereb Blood Flow Metab. 2008. PMID: 18612315 Free PMC article.
-
Selective CDK inhibitors: promising candidates for future clinical traumatic brain injury trials.Neural Regen Res. 2014 Sep 1;9(17):1578-80. doi: 10.4103/1673-5374.141779. Neural Regen Res. 2014. PMID: 25368642 Free PMC article. Review.
Cited by
-
CR8, a novel inhibitor of CDK, limits microglial activation, astrocytosis, neuronal loss, and neurologic dysfunction after experimental traumatic brain injury.J Cereb Blood Flow Metab. 2014 Mar;34(3):502-13. doi: 10.1038/jcbfm.2013.228. Epub 2014 Jan 8. J Cereb Blood Flow Metab. 2014. PMID: 24398934 Free PMC article.
-
Coming of Age: Targeting Cyclin K in Cancers.Cells. 2023 Aug 11;12(16):2044. doi: 10.3390/cells12162044. Cells. 2023. PMID: 37626854 Free PMC article. Review.
-
Cell cycle activation contributes to isoflurane-induced neurotoxicity in the developing brain and the protective effect of CR8.CNS Neurosci Ther. 2019 May;25(5):612-620. doi: 10.1111/cns.13090. Epub 2019 Jan 24. CNS Neurosci Ther. 2019. PMID: 30676695 Free PMC article.
-
Combined inhibition of cell death induced by apoptosis inducing factor and caspases provides additive neuroprotection in experimental traumatic brain injury.Neurobiol Dis. 2012 Jun;46(3):745-58. doi: 10.1016/j.nbd.2012.03.018. Epub 2012 Mar 9. Neurobiol Dis. 2012. PMID: 22426396 Free PMC article.
-
Neuroprotection for traumatic brain injury.Handb Clin Neurol. 2015;127:343-66. doi: 10.1016/B978-0-444-52892-6.00022-2. Handb Clin Neurol. 2015. PMID: 25702227 Free PMC article. Review.
References
-
- Faul M, Xu L, Wald M, Coronado V. Traumatic brain injury in the United States: Emergency department visits, hospitalizations and deaths 2002–2006. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control 2010;xx:xx-xx.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

