Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity
- PMID: 22167808
- PMCID: PMC3268324
- DOI: 10.1073/pnas.1118834109
Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity
Abstract
Infections with HIV, hepatitis B virus, and hepatitis C virus can turn into chronic infections, which currently affect more than 500 million patients worldwide. It is generally thought that virus-mediated T-cell exhaustion limits T-cell function, thus promoting chronic disease. Here we demonstrate that natural killer (NK) cells have a negative impact on the development of T-cell immunity by using the murine lymphocytic choriomeningitis virus. NK cell-deficient (Nfil3(-/-), E4BP4(-/-)) mice exhibited a higher virus-specific T-cell response. In addition, NK cell depletion caused enhanced T-cell immunity in WT mice, which led to rapid virus control and prevented chronic infection in lymphocytic choriomeningitis virus clone 13- and reduced viral load in DOCILE-infected animals. Further experiments showed that NKG2D triggered regulatory NK cell functions, which were mediated by perforin, and limited T-cell responses. Therefore, we identified an important role of regulatory NK cells in limiting T-cell immunity during virus infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

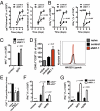


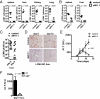
Comment in
-
Yet another role for natural killer cells: cytotoxicity in immune regulation and viral persistence.Proc Natl Acad Sci U S A. 2012 Feb 7;109(6):1814-5. doi: 10.1073/pnas.1120528109. Epub 2012 Jan 31. Proc Natl Acad Sci U S A. 2012. PMID: 22308452 Free PMC article. No abstract available.
References
-
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. - PubMed
-
- Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “L'union fait la force”. Blood. 2005;106:2252–2258. - PubMed
-
- Biron CA, Nguyen KB, Pien GC. Innate immune responses to LCMV infections: Natural killer cells and cytokines. Curr Top Microbiol Immunol. 2002;263:7–27. - PubMed
-
- Vivier E, Nunès JA, Vély F. Natural killer cell signaling pathways. Science. 2004;306:1517–1519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

