Improving the time-machine: estimating date of birth of grade II gliomas
- PMID: 22168136
- PMCID: PMC6496223
- DOI: 10.1111/j.1365-2184.2011.00790.x
Improving the time-machine: estimating date of birth of grade II gliomas
Abstract
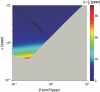
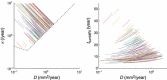
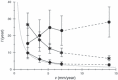
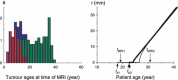
Objectives: Here we present a model aiming to provide an estimate of time from tumour genesis, for grade II gliomas. The model is based on a differential equation describing the diffusion-proliferation process. We have applied our model to situations where tumour diameter was shown to increase linearly with time, with characteristic diametric velocity.
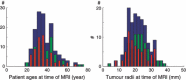
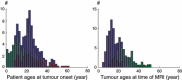
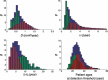
Materials and methods: We have performed numerical simulations to analyse data, on patients with grade II gliomas and to extract information concerning time of tumour biological onset, as well as radiology and distribution of model parameters.
Results and conclusions: We show that the estimate of tumour onset obtained from extrapolation using a constant velocity assumption, always underestimates biological tumour age, and that the correction one should add to this estimate is given roughly by 20/v (year), where v is the diametric velocity of expansion of the tumour (expressed in mm/year). Within the assumptions of the model, we have identified two types of tumour: the first corresponds to very slowly growing tumours that appear during adolescence, and the second type corresponds to slowly growing tumours that appear later, during early adulthood. That all these tumours become detectable around a mean patient age of 30 years could be interesting for formulation of strategies for early detection of tumours.
© 2011 Blackwell Publishing Ltd.
Figures

References
-
- Kelly PJ, Daumas‐Duport C, Kispert DB, Kall BA, Scheithauer W, Illig J (1987) Imaging‐based stereotaxic serial biopsies in untreated intracranial glial neaplasms. J. Neurosurg. 66, 865–874. - PubMed
-
- Pallud J, Varlet P, Devaux B, Geha S, Badoual M, Deroulers C et al. (2010) Diffuse low‐grade oligodendrogliomas extend beyond MRI‐defined abnormalities. Neurology 74, 1724–1731. - PubMed
-
- Keles GE, Lamborn KR, Berger MS (2001) Low‐grade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. J. Neurosurg. 95, 735–745. - PubMed
-
- Salgaller ML, Liau LM (2006) Current status of clinical trials for glioblastoma. Rev. Recent Clin. Trials 3, 265–281. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

