Effects of chronic carbon monoxide exposure on fetal growth and development in mice
- PMID: 22168775
- PMCID: PMC3297534
- DOI: 10.1186/1471-2393-11-101
Effects of chronic carbon monoxide exposure on fetal growth and development in mice
Abstract
Background: Carbon monoxide (CO) is produced endogenously, and can also be acquired from many exogenous sources: ie. cigarette smoking, automobile exhaust. Although toxic at high levels, low level production or exposure lends to normal physiologic functions: smooth muscle cell relaxation, control of vascular tone, platelet aggregation, anti- inflammatory and anti-apoptotic events. In pregnancy, it is unclear at what level maternal CO exposure becomes toxic to the fetus. In this study, we hypothesized that CO would be embryotoxic, and we sought to determine at what level of chronic CO exposure in pregnancy embryo/fetotoxic effects are observed.
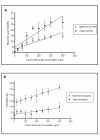
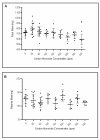
Methods: Pregnant CD1 mice were exposed to continuous levels of CO (0 to 400 ppm) from conception to gestation day 17. The effect on fetal/placental growth and development, and fetal/maternal CO concentrations were determined.
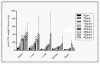
Results: Maternal and fetal CO blood concentrations ranged from 1.12- 15.6 percent carboxyhemoglobin (%COHb) and 1.0- 28.6%COHb, respectively. No significant difference was observed in placental histological morphology or in placental mass with any CO exposure. At 400 ppm CO vs. control, decreased litter size and fetal mass (p < 0.05), increased fetal early/late gestational deaths (p < 0.05), and increased CO content in the placenta and the maternal spleen, heart, liver, kidney and lung (p < 0.05) were observed.
Conclusions: Exposure to levels at or below 300 ppm CO throughout pregnancy has little demonstrable effect on fetal growth and development in the mouse.
Figures


References
-
- Brune B, Ullrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol Pharmacol. 1987;32:497–504. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

