Peroxisome proliferator-activated receptor-γ agonists repress epithelial sodium channel expression in the kidney
- PMID: 22169011
- PMCID: PMC3353644
- DOI: 10.1152/ajprenal.00306.2011
Peroxisome proliferator-activated receptor-γ agonists repress epithelial sodium channel expression in the kidney
Abstract
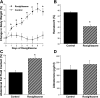
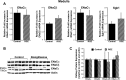
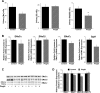
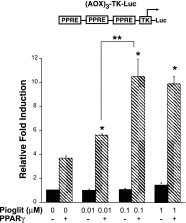
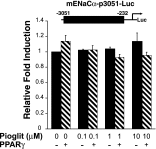
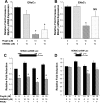
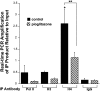
Thiazolidinediones (TZDs), known as peroxisome proliferator-activated receptor (PPAR) agonists, are used to treat type 2 diabetes. However, ∼5% of patients experience the treatment-limiting side effect of edema. Studies have implicated activation of the epithelial sodium channel (ENaC) as a cause of TZD-induced fluid retention, although there have been conflicting reports. The goal of this study was to resolve the role of PPARγ in control of ENaC isoforms in the kidney. Herein, we demonstrate in mice that rosiglitazone (RGZ), a PPARγ ligand, increases body weight and abdominal fat pad fluid content and reduces hematocrit. Seven days of RGZ decreases ENaCα and ENaCβ mRNA and ENaCγ protein expression in the kidney cortex, and acute treatment for 5 h with pioglitazone, another potent TZD, does not increase renal ENaC isoform mRNA or protein expression. Pioglitazone also decreases ENaCα and ENaCγ mRNA expression in a cortical collecting duct cell line. As no direct transcriptional studies had been conducted, we examined the PPARγ-dependent regulation of ENaC. Pioglitazone represses ENaCγ promoter activity, and this repression is partially relieved by inhibition of protein synthesis. Chromatin immunoprecipitation assays revealed that repression is associated with a decrease in histone H4K5 acetylation at the proximal ENaCγ promoter. In summary, TZDs do not increase ENaC mRNA expression in the kidney, and in fact repress the ENaCγ promoter via an indirect transcriptional mechanism.
Figures



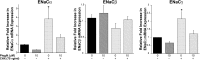
References
-
- Artunc F, Sandulache D, Nasir O, Boini KM, Friedrich B, Beier N, Dicks E, Potzsch S, Klingel K, Amann K, Blazer-Yost BL, Scholz W, Risler T, Kuhl D, Lang F. Lack of the serum and glucocorticoid-inducible kinase SGK1 attenuates the volume retention after treatment with the PPARgamma agonist pioglitazone. Pflügers Arch 456: 425–436, 2008. - PubMed
-
- Cunard R, Eto Y, Muljadi JT, Glass CK, Kelly CJ, Ricote M. Repression of IFN-gamma expression by peroxisome proliferator-activated receptor gamma. J Immunol 172: 7530–7536, 2004. - PubMed
-
- Dagenais A, Denis C, Vives MF, Girouard S, Masse C, Nguyen T, Yamagata T, Grygorczyk C, Kothary R, Berthiaume Y. Modulation of α-ENaC and α1-Na+-K+-ATPase by cAMP and dexamethasone in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 281: L217–L230, 2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

