Extended safety and efficacy studies of the attenuated Brucella vaccine candidates 16 M(Delta)vjbR and S19(Delta)vjbR in the immunocompromised IRF-1-/- mouse model
- PMID: 22169089
- PMCID: PMC3272933
- DOI: 10.1128/CVI.05321-11
Extended safety and efficacy studies of the attenuated Brucella vaccine candidates 16 M(Delta)vjbR and S19(Delta)vjbR in the immunocompromised IRF-1-/- mouse model
Abstract
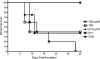
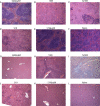
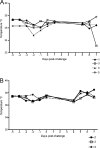
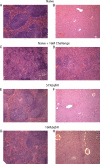
The global distribution of brucellosis and high incidence in certain areas of the world warrant the development of a safer and efficacious vaccine. For the past 10 years, we have focused our attention on the development of a safer, but still highly protective, live attenuated vaccine for human and animal use. We have demonstrated the safety and protective efficacy of the vaccine candidates 16 MΔvjbR and S19ΔvjbR against homologous and heterologous challenge in multiple immunocompetent animal models, including mice and deer. In the present study, we conducted a series of experiments to determine the safety of the vaccine candidates in interferon regulatory factor-1-knockout (IRF-1(-/-)) mice. IRF-1(-/-) mice infected with either wild-type Brucella melitensis 16 M or the vaccine strain Brucella abortus S19 succumb to the disease within the first 3 weeks of infection, which is characterized by a marked granulomatous and neutrophilic inflammatory response that principally targets the spleen and liver. In contrast, IRF-1(-/-) mice inoculated with either the 16 MΔvjbR or S19ΔvjbR vaccine do not show any clinical or major pathological changes associated with vaccination. Additionally, when 16 MΔvjbR- or S19ΔvjbR-vaccinated mice are challenged with wild-type Brucella melitensis 16M, the degree of colonization in multiple organs, along with associated pathological changes, is significantly reduced. These findings not only demonstrate the safety and protective efficacy of the vjbR mutant in an immunocompromised mouse model but also suggest the participation of lesser-known mechanisms in protective immunity against brucellosis.
Figures





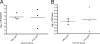
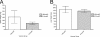
References
-
- Arenas-Gamboa AM, Ficht TA, Kahl-McDonagh MM, Gomez G, Rice-Ficht AC. 2009. The Brucella abortus S19 deltavjbR live vaccine candidate is safer than S19 and confers protection against wild-type challenge in BALB/c mice when delivered in a sustained-release vehicle. Infect. Immun. 77:877–884 - PMC - PubMed
-
- Clemmer TP, et al. 1992. Hypothermia in the sepsis syndrome and clinical outcome. The Methylprednisolone Severe Sepsis Study Group. Crit. Care Med. 20:1395–1401 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

