Optimization of in vivo confocal autofluorescence imaging of the ocular fundus in mice and its application to models of human retinal degeneration
- PMID: 22169101
- PMCID: PMC3317405
- DOI: 10.1167/iovs.11-8767
Optimization of in vivo confocal autofluorescence imaging of the ocular fundus in mice and its application to models of human retinal degeneration
Abstract
Purpose: To investigate the feasibility and to identify sources of experimental variability of quantitative and qualitative fundus autofluorescence (AF) assessment in mice.
Methods: Blue (488 nm) and near-infrared (790 nm) fundus AF imaging was performed in various mouse strains and disease models (129S2, C57Bl/6, Abca4(-/-), C3H-Pde6b(rd1/rd1), Rho(-/-), and BALB/c mice) using a commercially available scanning laser ophthalmoscope. Gray-level analysis was used to explore factors influencing fundus AF measurements.
Results: A contact lens avoided cataract development and resulted in consistent fundus AF recordings. Fundus illumination and magnification were sensitive to changes of the camera position. Standardized adjustment of the recorded confocal plane and consideration of the pupil area allowed reproducible recording of fundus AF from the retinal pigment epithelium with an intersession coefficient of repeatability of ±22%. Photopigment bleaching occurred during the first 1.5 seconds of exposure to 488 nm blue light (∼10 mW/cm(2)), resulting in an increase of fundus AF. In addition, there was a slight decrease in fundus AF during prolonged blue light exposure. Fundus AF at 488 nm was low in animals with an absence of a normal visual cycle, and high in BALB/c and Abca4(-/-) mice. Degenerative alterations in Pde6b(rd1/rd1) and Rho(-/-) were reminiscent of findings in human retinal disease.
Conclusions: Investigation of retinal phenotypes in mice is possible in vivo using standardized fundus AF imaging. Correlation with postmortem analysis is likely to lead to further understanding of human disease phenotypes and of retinal degenerations in general. Fundus AF imaging may be useful as an outcome measure in preclinical trials, such as for monitoring effects aimed at lowering lipofuscin accumulation in the retinal pigment epithelium.
Figures


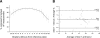
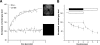


References
-
- Rivas MA, Vecino E. Animal models and different therapies for treatment of retinitis pigmentosa. Histol Histopathol. 2009;24:1295–1322 - PubMed
-
- Paques M, Simonutti M, Roux M, et al. High resolution fundus imaging by confocal scanning laser ophthalmoscopy in the mouse. Vision Res. 2006;46:1336–1345 - PubMed
-
- Seeliger MW, Beck SC, Pereyra-Muñoz N, et al. In vivo confocal imaging of the retina in animal models using scanning laser ophthalmoscopy. Vision Res. 2005;45:3512–3519 - PubMed
-
- Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging: review and perspectives. Retina. 2008;28:385–409 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

